Abstract
We examined the cerebellum and cerebrum of 4 vaccinated dogs, 3–60-mo-old, that displayed clinical signs of canine distemper virus (CDV) infection, and died 7–40 d after developing neurologic signs. The main histologic lesions were demyelination, gliosis, meningitis, perivascular lymphocytic cuffing, and inclusion bodies. These lesions were similar in all 4 cases regardless of the time since vaccination, except that meningoencephalitis and gliosis were subacute in 3 dogs and chronic in 1 dog. However, these differences did not appear to be related to their vaccination status. Immunohistologically, a CDV-positive immunoreaction was seen mainly in astrocytes, neurons and their axons, lymphocytes around and in the blood vessels of the pia mater and choroid plexus, ependymal cells of each ventricle, and the cells of the choroid plexus. The histologic and immunohistologic changes were similar in the cerebellum and cerebrum. The genetic characterization of the virus strains in 2 of these naturally occurring canine distemper cases confirmed that they were South American wild-type strains (Kiki and Uy251) belonging to the EU1/SA1 lineage. These strains are not included in the commercial CDV vaccines available in Uruguay.
Keywords: canine distemper virus, demyelination, dogs, pathology, vaccination
Canine distemper virus (CDV; Paramyxoviridae, Canine morbillivirus) causes one of the most common infectious diseases of domestic dogs. CDV is also known to infect various other non-canine hosts sporadically, including a wide range of terrestrial and marine carnivores. Although commercial CDV vaccines have been available since the 1950s, there have been reports of cases of CDV in vaccinated dogs, and the incidence of CDV-induced disease is reported to be increasing in vaccinated dogs throughout the world.2,3,5,6,10,15,16,18,25 There has been much discussion regarding these vaccine breaks or CDV-induced disease in vaccinated dogs. Suggested reasons include improper handling of the vaccine that may reduce its effectiveness, maternal antibodies that may interfere with vaccine immunogenicity, or there may be antigenic differences among wild-type CDV strains and commercial vaccine strains.2,3,7,14,16
In a 5-y study (2008–2012) in Brazil, of 155 dogs that developed CDV-induced disease, 17 (12.2%) were vaccinated, 15 (9.6%) were not properly revaccinated or given boosters, 76 (49%) were unvaccinated, and the vaccine status of the rest was unknown. 3 These findings suggest that CDV-associated neurologic disease in dogs occurs in South America even though affected dogs have been vaccinated. There have been reports of CDV-induced disease in vaccinated dogs in other parts of the world, involving various vaccines, vaccines from different producers, and genetically diverse dogs.5,6,10 Severe nonsuppurative leptomeningitis, with multifocal demyelination and hemorrhages, has been described in vaccinated adult dogs in North America. 6 Encephalitis, with gliosis, typical eosinophilic CDV inclusion bodies, and minimal demyelination, has been described in New Zealand in Border Collie-cross littermates that were reported to have been vaccinated. 5 Neither report5,6 compared the effect of vaccination, such as vaccine titers or the time since vaccination. More information is needed to better understand CDV-induced disease and its relationship to vaccination or animal immunocompetence.
We aimed to better characterize the pathologic changes in 4 CDV-vaccinated dogs with CDV-associated neurologic disease in Uruguay. We analyzed the distribution and characteristics of the CDV-induced lesions and the age and vaccination status of the dogs.
Materials and methods
Dogs
Case 1 was a 3-mo-old female Maltese dog that had been vaccinated by a veterinarian with commercial CDV attenuated live vaccine (Providean Viratec 6CV; Tecnovax) at 45 and 70 d old. Twelve days after the last vaccination, the dog developed diarrhea and fever. This was followed by coughing, nasal and ocular mucopurulent discharge, and footpad hyperkeratosis. Myoclonus, ataxia, and paresis appeared 20 d post-vaccination (dpv; Table 1). Neurologic disease became more severe, and the dog died 27 dpv.
Table 1.
Clinical signs and histologic and immunohistologic changes consistent with canine distemper in 4 vaccinated dogs.
| Case, sex, age | Vaccination | Neurologic signs | Days showing neurologic signs before death | Histopathologic and immunohistologic findings | ||
|---|---|---|---|---|---|---|
| Cerebellum | Cerebrum | Stage | ||||
| 1 F, 3 mo | On vaccination plan | Myoclonus, ataxia, paresis | 7 | Demyelination, gliosis, meningitis, IB, cortical necrosis, CDV particles | Demyelination, gliosis, meningitis, IB, CDV particles | Subacute |
| 2 M, 13 mo | On vaccination plan | Myoclonus, nystagmus, quadriplegia | 40 | Demyelination, gliosis, meningitis, cuffing, IB, cortical necrosis, CDV particles | Demyelination, gliosis, meningitis, cuffing, IB, CDV particles | Chronic |
| 3 F, 24 mo | On vaccination plan | Myoclonus, paresis | 8 | Demyelination, gliosis, meningitis, cuffing, IB, CDV particles | Demyelination, gliosis, meningitis, cuffing, IB, CDV particles | Subacute |
| 4 M, 60 mo | Out of vaccination plan | Myoclonus, seizures, circling, compulsive gait, stupor | 25 | Demyelination, gliosis, meningitis, cuffing, CDV particles | Demyelination, gliosis, meningitis, cuffing, CDV particles | Subacute |
F = female; IB = inclusion bodies; M = male.
Case 2 was a 13-mo-old male, mixed-breed dog, reported to have been vaccinated by a veterinarian according to the recommended protocol with initial immunization followed by a booster 21 d later (Recombitek C6; Boehringer-Ingelheim). This dog also developed nasal mucopurulent discharge as well as footpad and nose hyperkeratosis ~40 dpv. Severe neurologic disease appeared at 40 dpv, and the dog died ~55 dpv.
Case 3 was a 24-mo-old female Pitbull Terrier. The dog was reported to have been regularly vaccinated by a veterinarian with a yearly booster after the initial sequence of commercial CDV attenuated live virus vaccine (the commercial vaccine name was not given in the history). This dog developed signs of distemper, including dyspnea and coughing, mucopurulent nasal discharge, and footpad hyperkeratosis. The dog developed myoclonus and paresis and died 25 d later.
Case 4 was a 60-mo-old male Labrador Retriever that was vaccinated by a veterinarian with yearly boosters of commercial CDV attenuated live virus vaccines until 3 y old (the commercial vaccine names were not given in the history). Nearly 2 y later, the animal developed diarrhea, vomiting, and nasal hyperkeratosis. Myoclonus, seizures, circling, compulsive gait, and stupor developed, and the dog died 25 d later.
Histopathology and immunohistochemistry
An autopsy was performed on all 4 dogs, and selected tissues, including the brain and lungs, were fixed in 10% neutral-buffered formalin and processed routinely for histologic examination. Tissue sections were stained with hematoxylin & eosin and Luxol fast blue (LFB) stains. For immunohistochemistry (IHC), a mouse anti-CDV monoclonal primary antibody (MCA 1893, dilution 1:250; Bio-Rad) was used to detect CDV antigens. The primary antibody was incubated overnight at 4°C followed by HRP-polymer incubation in two 10-min steps, at room temperature, according to the manufacturer’s instructions (Mouse-on-canine HRP-polymer; Biocare Medical). Positive antigen–antibody reactions were observed by incubation with 3,3′-diaminobenzidine tetrahydrochloride (DAB), as described previously. 5 Slides were counterstained with Mayer hematoxylin and coverslipped.
The stages of the histologic lesions were categorized as acute, subacute, or chronic based on previous studies.1,8,11,19-24 Briefly, acute lesions were characterized by focal vacuolation, minimal gliosis, minimal perivascular cuffing and inclusion bodies, and the presence of CDV-positive cells. Subacute lesions included patchy demyelination, extensive gliosis, neuronal necrosis, prominent perivascular cuffing (2–3 layers of mononuclear inflammatory cells), prominent CDV inclusion bodies, and numerous CDV-positive cells. Chronic lesions were similar to those in the subacute stage but had increased neuronal degeneration and necrosis with perivascular infiltration of at least 3 layers.
Amplification of the Fsp-coding region and genetic characterization of the CDV strains
Brain samples from the dogs were subjected to TRIzol treatment (Invitrogen, Life Technologies) to isolate CDV RNA. The first-strand complementary DNA was synthesized in cases 2 and 3 (SensiFast cDNA synthesis kit; Bioline), according to the manufacturer’s instructions. Specific primers were used to amplify the Fsp-coding region (681 bp). The cycling conditions and primers, F4854: TCCAGGACATAGCAAGCCAACA and R5535: GGTTGATTGGTTCGAGGACTGAA, were used according to a previous report. 18 The PCR products were sequenced (Macrogen, Seoul, Korea), and the sequences were aligned using BLAST (https://blast.ncbi.nlm.nih.gov/Blast.cgi).
Results
The main histologic lesions were demyelination, gliosis, meningitis, perivascular cuffing, and inclusion bodies (Table 1). There were no qualitative or quantitative differences between the cerebrum and cerebellum.
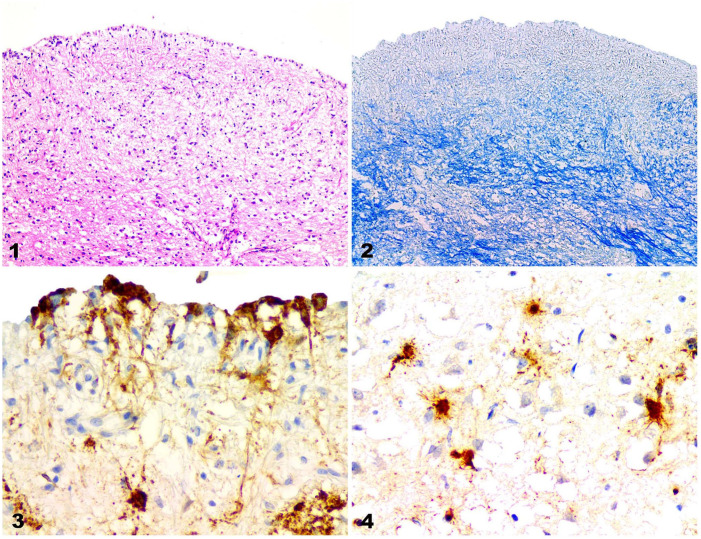
In all of the dogs, the most consistent change was demyelination (Fig. 1), which was easily assessed using LFB staining (Fig. 2). In the demyelinated areas, many astrocytes contained CDV-positive particles (Figs. 3, 4). Demyelination was most severe in periventricular white matter tracts and submeningeal neuropil; gliosis, particularly astrocytic gliosis, was commonly found nearby. Meningitis involving the pia mater was observed in all cases. Many of the mononuclear inflammatory cells with anti-CDV immunoreaction were detected in and around blood vessels in the pia mater; many more were present throughout the demyelinated and necrotic foci.
Figures 1–4.
Canine distemper nervous system lesions in vaccinated dogs. Figure 1. Demyelination in the periventricular white matter of the cerebrum in case 4. H&E. Original objective 10×. Figure 2. Demyelination in the cerebrum of case 4. Same area as Figure 1. LFB. Original objective 10×. Figure 3. CDV-immunopositive cells in ependymal and other cells in the cerebrum of case 4. IHC against CDV. Original objective 40×. Figure 4. CDV-immunopositive particles in astrocytes in a demyelinated lesion in the cerebrum in case 1. IHC against CDV. Original objective 40×.
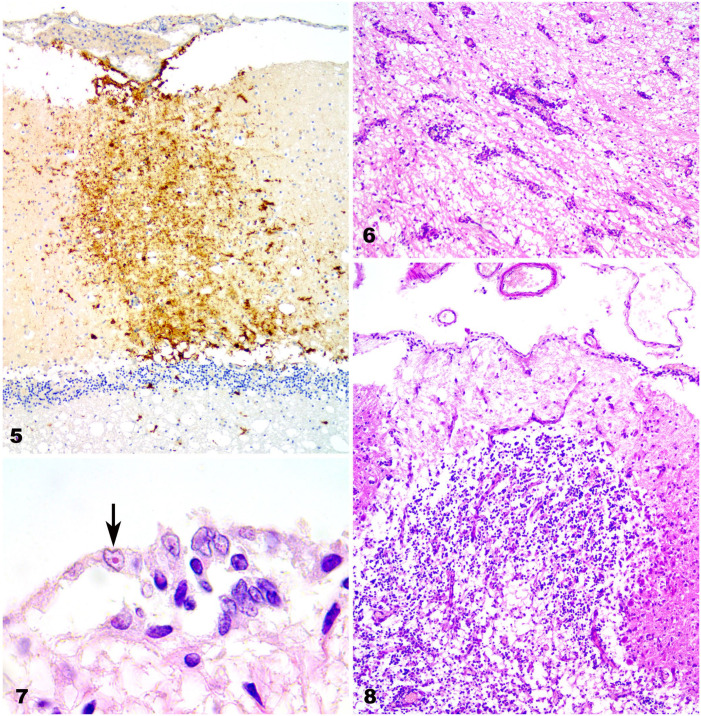
Cerebral and cerebellar necrosis with nonsuppurative meningitis was common in dogs 1 and 2 (Table 1). These animals also had intense submeningeal anti-CDV immunoreactivity that extended from the pia mater deep into the cortex. A similar immunoreaction was detected in various neurons, including cerebellar cortical neurons (Fig. 5). Perivascular cuffing was prominent in 3 cases. The perivascular lymphocytic infiltration was 2–3 layers thick in cases 3 and 4, and was more extensive, with >3 layers of thickness in case 2 (Fig. 6). Intranuclear inclusion bodies were detected in glia, ependymal cells, and neurons (Fig. 7); however, inclusion bodies were most common in astrocytes. From these findings, cases 1, 3, and 4 were considered to be in the subacute stage, and case 2 in the chronic stage.
Figures 5–8.
Canine distemper nervous system lesions in vaccinated dogs. Figure 5. CDV-immunopositive cells in the pia matter and throughout the molecular layer in the cerebellar cortex of case 1. IHC against CDV. Original objective 10×. Figure 6. Perivascular lymphocytic cuffing in the cerebrum of case 2. H&E. Original objective 10×. Figure 7. Intranuclear inclusion bodies in ependymal cells (arrow) in the cerebrum of case 4. High magnification of Figure 1. H&E. Original objective 100×. Figure 8. Necrotic area with Purkinje cell loss in the cerebellum of case 2. H&E. Original objective 40×.
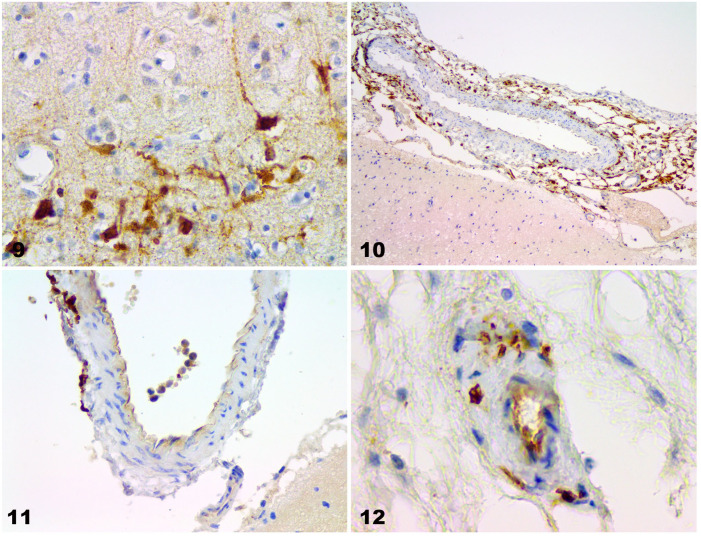
Animals that had severe white matter demyelination and cortical necrosis also had decreased numbers of Purkinje cells (Fig. 8). In the cerebral cortex, there was intense anti-CDV immunoreactivity, both in neurons and their dendrites and axons. There were patches of degenerate neurons with anti–CDV-positive immunoreactivity in the cerebellum and cerebrum (Fig. 9). Anti-CDV immunoreactivity was also positive in epithelial cells and blood vessels of the choroid plexus. Furthermore, some mononuclear cells were CDV positive around and in the blood vessels of the pia mater and the parenchyma (Figs. 10–12).
Figures 9–12.
Canine distemper nervous system lesions in vaccinated dogs. Figure 9. CDV-immunopositive particles in nerve cell axons and astrocytes in the cerebrum of case 4. IHC against CDV. Original objective 40×. Figure 10. Perivascular CDV-immunopositive cells in the cerebral pia mater in case 1. IHC against CDV. Original objective 10×. Figure 11. Intravascular and perivascular CDV-immunopositive cells in the cerebral pia mater in case 1. IHC against CDV. Original objective 40×. Figure 12. CDV-immunopositive cells in endothelial cells and in the blood vessel wall in the cerebellum in case 4. IHC against CDV. Original objective 40×.
The Fsp-coding region sequences obtained from the brains of cases 2 and 3 were aligned and compared to sequences in the NCBI database. The sequence of case 2 (histologically classified as chronic) had nucleotide similarity of 96.2% to the CDV isolate Kiki (Table 2). The sequence of case 3 (subacute) was 98.5% similar to CDV strain Uy251 (Table 3).
Table 2.
BLAST alignment of the Fsp-coding region from case 2 with existing sequences (NCBI database) and nucleotide similarity percentage (NSP).
| Description | Max score | Total score | Query cover | E-value | NSP | Accession |
|---|---|---|---|---|---|---|
| Canine morbillivirus isolate CDV Kiki, complete genome | 959 | 1,734 | 47% | 0.0 | 96.2* | MH484613.1 |
| Canine morbillivirus strain BRA/UEL-05/03 fusion protein gene, partial cds | 942 | 1,712 | 47% | 0.0 | 95.7 | KY057345.1 |
| Canine morbillivirus strain BRA/UEL-MEG/14 fusion protein gene, partial cds | 937 | 1,715 | 46% | 0.0 | 95.6 | KY057353.1 |
| Canine morbillivirus strain BRA/UEL-18/03 fusion protein gene, partial cds | 937 | 1,704 | 46% | 0.0 | 95.6 | KY057349.1 |
| Canine morbillivirus strain BRA/UEL-15/03 fusion protein gene, partial cds | 937 | 1,695 | 47% | 0.0 | 95.6 | KY057347.1 |
| Canine distemper virus strain Uy251, complete genome | 937 | 1,706 | 47% | 0.0 | 95.6 | KM280689.1 |
cds = coding sequences.
High homology with the CDV isolate Kiki (Brazilian strain belonging to the EU1/SA1 lineage).
Table 3.
BLAST alignment of the Fsp-coding region from case 3 with the previously determined sequences (NCBI database) and nucleotide similarity percentage (NSP).
| Description | Max score | Total score | Query cover | E-value | NSP | Accession |
|---|---|---|---|---|---|---|
| Canine distemper virus strain Uy251, complete genome | 848 | 848 | 21% | 0.0 | 98.5* | KM280689.1 |
| Canine morbillivirus strain BRA/UEL-MEG/14 fusion protein gene, partial cds | 815 | 815 | 21% | 0.0 | 96.9 | KY057353.1 |
| Canine morbillivirus strain BRA/UEL-LLK/16 fusion protein gene, partial cds | 809 | 809 | 21% | 0.0 | 96.7 | KY057357.1 |
| Canine morbillivirus strain BRA/UEL-ZNH/16 fusion protein gene, partial cds | 809 | 809 | 21% | 0.0 | 96.7 | KY057356.1 |
| Canine morbillivirus strain BRA/UEL-05/03 fusion protein gene, partial cds | 808 | 808 | 22% | 0.0 | 96.5 | KY057345.1 |
| Canine morbillivirus strain BRA/UEL-TCO/16 fusion protein gene, partial cds | 804 | 804 | 21% | 0.0 | 96.5 | KY057358.1 |
| Canine morbillivirus strain BRA/UEL-LLA/14 fusion protein gene, partial cds | 798 | 798 | 21% | 0.0 | 96.3 | KY057354.1 |
cds = coding sequences.
High homology with the canine distemper virus isolate Uy251 (Uruguayan strain belonging to the EU1/SA1 lineage).
Discussion
Histologically, the main lesions in our cases were demyelination, gliosis, meningoencephalitis, and inclusion bodies, consistent with a previous report. 10 Unlike a previous report, 6 we did not observe hemorrhages. Our findings are similar to the basic histologic changes in an experimental infection study using specific pathogen–free dogs. 21
We classified 3 cases as subacute and 1 case as chronic. The neurologic signs developed over 7–25 and 40 d in the subacute and chronic cases, respectively. Therefore, we concluded that there was an association between the clinical signs and the histologic classification. In a report of 21 naturally infected CDV dogs, cerebellar lesions were acute in 12, subacute in 5, and chronic in 4 dogs; 12 of these dogs died naturally, and 9 were euthanized. In the group of dogs that died naturally, all were found to have acute lesions. In another report, among 43 dogs infected with CDV, 11 (26%) dogs had acute encephalopathy, 4 (9%) had acute lesions with necrotic changes, 22 (51%) had subacute encephalitis, and 6 (14%) had chronic encephalitis.9,24
The 4 dogs that we examined all died naturally, without findings of the acute histologic stage. This may be because the dogs in our study were vaccinated. The differences, including the histologic stages between the unvaccinated and vaccinated dogs, are suggested to be important in considering the usefulness of vaccines, hence further investigation is needed. Furthermore, our cases differed from post-vaccinal CDV disease, which characteristically occurs in young dogs 1–3 wk after vaccination with commercial attenuated CDV vaccines.4,5 Post-vaccinal CDV disease is typically characterized by acute-to-subacute clinical presentation (1–5 d) or is seen as “old dog” encephalitis, which occurs as a consequence of long-term, subclinical, persistent CDV infection. 4
The invasion of CDV into the brain from other organs has been examined in previous reports.11,14 The main route of invasion is via infected lymphocytes trafficking through the blood–brain barrier; virus infects resident epithelial and endothelial cells. 11 CDV-infected cells are first detected in the choroid vessels, the surrounding pia mater, and the choroid plexus cells. Subsequently, virus spreads from the cells of the pia mater to the subpial gray matter. 14 Once inside the brain, the virus is proposed to spread via the cerebrospinal fluid and may infect the ependymal lining cells of the ventricles and, ultimately, glia and neurons. In our immunohistologic results, the ependymal cells and endothelial cells of the blood vessels in the choroid plexus and pia mater were immunopositive to the anti-CDV antibody. Furthermore, CDV-immunopositive mononuclear cells were detected intravascularly or perivascularly in the pia mater and brain parenchyma. In addition, severe demyelination was evident in the periventricular and subcortical white matter and co-localized with CDV antigen. These findings support movement of the virus from the blood vessels into the brain parenchyma. Necrosis of cerebellar white matter with meningitis was prominent in 2 cases, and these cases were accompanied by high CDV immunoreactivity from the pia mater to the deep white matter. This suggests that CDV entered the cerebellar cortex directly from the pia mater affected with meningitis, resulting in cortical necrosis. A CDV-positive immunoreaction in astrocytes was one of the characteristic findings in our study; the presence of CDV in many astrocytes is thought to be related to the mechanism of demyelination.11-13
With reduced or incomplete vaccination in domestic dog populations, 16 as occurs frequently in some South American countries, herd immunity could be lower, increasing classical CDV cases in previously vaccinated dogs because of increased exposure. 16 In Brazil 3 and Uruguay,17,18 some specific CDV circulating wild-type strains were genotyped. All of them were found to belong to the EU1/SA1 clade and had important antigenic differences from CDV strains included in commercial vaccines.2,16 Our results confirm these reports.3,17,18 In our cases, 2 wild-type strains acting on the central nervous system in vaccinated dogs were found. Consistent with a previous report, 5 CDV disease in our cases was caused by wild-type strains not included in commercial vaccines, and not by post-vaccinal CDV disease. Our results highlight the relevance of CDV infection in Uruguay, the circulation of at least 2 wild-type CDV strains that are not included in commercial vaccines, the need to intensify current control measures, 3 and the need for specific CDV commercial vaccines that include South American wild-type CDV strains.
Acknowledgments
Gimena Feijóo is the recipient of a PhD fellowship from ANII, and José Manuel Verdes is a Research Career Member of the National Research System (SNI-ANII), Uruguay. We thank Camila Larrañaga and Victoria Iribarnegaray for their technical assistance and Bryan Stegelmeier for critically reviewing and editing this manuscript in the English language.
Footnotes
Declaration of conflicting interests: The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Funding: This work was supported by the National Agency of Research and Innovation (ANII Fondo María Viñas 2019, grant FMV-1-2019-1-155934), the Committee of Scientific Research of the University of the Republic (CSIC, UdelaR), and the Basic Science Development Program (PEDECIBA), Uruguay.
ORCID iD: José Manuel Verdes  https://orcid.org/0000-0003-4314-906X
https://orcid.org/0000-0003-4314-906X
Contributor Information
Gimena Feijóo, Departments of Pathobiology, Veterinary Faculty, Universidad de la República, Montevideo, Uruguay; Veterinary Clinics & Hospital, Veterinary Faculty, Universidad de la República, Montevideo, Uruguay.
Kanji Yamasaki, Departments of Pathobiology, Veterinary Faculty, Universidad de la República, Montevideo, Uruguay.
Luis Delucchi, Veterinary Clinics & Hospital, Veterinary Faculty, Universidad de la República, Montevideo, Uruguay.
José Manuel Verdes, Departments of Pathobiology, Veterinary Faculty, Universidad de la República, Montevideo, Uruguay.
References
- 1. Alldinger S, et al. Up-regulation of the hyaluronate receptor CD44 in canine distemper demyelinated plaques. Acta Neuropathol 2000;99:138–146. [DOI] [PubMed] [Google Scholar]
- 2. Anis E, et al. Antigenic analysis of genetic variants of canine distemper virus. Vet Microbiol 2018;219:154–160. [DOI] [PubMed] [Google Scholar]
- 3. Budaszewski RF, et al. Genotyping of canine distemper virus strains circulating in Brazil from 2008 to 2012. Virus Res 2014;180:76–83. [DOI] [PubMed] [Google Scholar]
- 4. Cantile C, Youssef S. Nervous system. In: Maxie MG, ed. Jubb, Kennedy, and Palmer’s Pathology of Domestic Animals. Vol. 1. 6th ed. Elsevier, 2016:250–406. [Google Scholar]
- 5. Fairley RA, et al. Post-vaccinal distemper encephalitis in two Border Collie cross littermates. N Z Vet J 2015;63:117–120. [DOI] [PubMed] [Google Scholar]
- 6. Galán A, et al. Uncommon acute neurologic presentation of canine distemper in 4 adult dogs. Can Vet J 2014;55:373–378. [PMC free article] [PubMed] [Google Scholar]
- 7. Garigliany M, et al. Re-emergence of canine distemper in wildlife in Belgium. Vet Rec 2018;182:439. [DOI] [PubMed] [Google Scholar]
- 8. Gröters S, et al. Up-regulation of mRNA for matrix metalloproteinases-9 and -14 in advanced lesions of demyelinating canine distemper leukoencephalitis. Acta Neuropathol 2005;110:369–382. [DOI] [PubMed] [Google Scholar]
- 9. Headley SA, et al. Glial fibrillary acidic protein (GFAP)-immunoreactive astrocytes in dogs infected with canine distemper virus. J Comp Pathol 2001;125:90–97. [DOI] [PubMed] [Google Scholar]
- 10. Lan NT, et al. Comparative analyses of canine distemper viral isolates from clinical cases of canine distemper in vaccinated dogs. Vet Microbiol 2006;115:32–42. [DOI] [PubMed] [Google Scholar]
- 11. Lempp C, et al. New aspects of the pathogenesis of canine distemper leukoencephalitis. Viruses 2014;6:2571–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mutinelli F, et al. Astrocytic infection in canine distemper virus-induced demyelination. Acta Neuropathol 1989;77:333–335. [DOI] [PubMed] [Google Scholar]
- 13. Pan Y, et al. Pathogenesis of demyelinating encephalopathy in dogs with spontaneous acute canine distemper. J Integr Agric 2013;12:334–343. [Google Scholar]
- 14. Rendon-Marin S, et al. Tropism and molecular pathogenesis of canine distemper virus. Virol J 2019;16:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Richards TR, et al. Optic neuritis caused by canine distemper virus in a Jack Russell terrier. Can Vet J 2011;52:398–402. [PMC free article] [PubMed] [Google Scholar]
- 16. Riley MC, Wilkes RP. Sequencing of emerging canine distemper virus strain reveals new distinct genetic lineage in the United States associated with disease in wildlife and domestic canine populations. Virol J 2015;12:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sarute N, et al. First genome sequence of a canine distemper virus strain from South America. Genome Announc 2014;2:e01009-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sarute N, et al. The fusion protein signal-peptide-coding region of canine distemper virus: a useful tool for phylogenetic reconstruction and lineage identification. PLoS One 2013;13:e63595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Seehusen F, et al. Vimentin-positive astrocytes in canine distemper: a target for canine distemper virus especially in chronic demyelinating lesions? Acta Neuropathol 2007;114:597–608. [DOI] [PubMed] [Google Scholar]
- 20. Silva MC, et al. Neuropathology of canine distemper: 70 cases (2005–2008) [Neuropatologia da Cinomose canina: 70 casos (2005–2008)]. Pesq Vet Bras 2009;29:643–652. Portuguese. [Google Scholar]
- 21. Spitzbarth I, et al. Immunohistochemical and transcriptome analyses indicate complex breakdown of axonal transport mechanisms in canine distemper leukoencephalitis. Brain Behav 2016;3:e00472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Summers BA, et al. Early events in canine distemper demyelinating encephalomyelitis. Acta Neuropathol 1979;46:1–10. [DOI] [PubMed] [Google Scholar]
- 23. Ulrich R, et al. Transcriptional changes in canine distemper virus-induced demyelinating leukoencephalitis favor a biphasic mode of demyelination. PLoS One 2014;9:e95917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vandevelde M, et al. Immunoglobulins in demyelinating lesions in canine distemper encephalitis. An immunohistological study. Acta Neuropathol 1981;54:31–41. [DOI] [PubMed] [Google Scholar]
- 25. Zhao J, et al. Emergence of canine distemper virus strains with two amino acid substitutions in the haemagglutinin protein, detected from vaccinated carnivores in north-eastern China in 2012–2013. Vet J 2014;200:191–194. [DOI] [PubMed] [Google Scholar]