Abstract
Owing to the richness of bioactive compounds, Olea europea leaf extracts exhibit a range of health effects. The present research evaluated the antibacterial and antiviral effect of leaf extracts obtained from Olea europea L. var. sativa (OESA) and Olea europea var. sylvestris (OESY) from Tunisia. LC-DAD-ESI-MS analysis allowed the identification of different compounds that contributed to the observed biological properties. Both OESA and OESY were active against Gram-positive bacteria (MIC values between 7.81 and 15.61 μg/mL and between 15.61 and 31.25 μg/mL against Staphylococcus aureus ATCC 6538 for OESY and OESA, respectively). The antiviral activity against the herpes simplex type 1 (HSV-1) was assessed on Vero cells. The results of cell viability indicated that Olea europea leaf extracts were not toxic to cultured Vero cells. The half maximal cytotoxic concentration (CC50) values for OESA and OESY were 0.2 mg/mL and 0.82 mg/mL, respectively. Furthermore, both a plaque reduction assay and viral entry assay were used to demonstrate the antiviral activity. In conclusion, Olea europea leaf extracts demonstrated a bacteriostatic effect, as well as remarkable antiviral activity, which could provide an alternative treatment against resistant strains.
Keywords: Olea europea L., LC-DAD-ESI-MS analysis, antimicrobial effect, antiviral activity, herpes simplex virus 1
1. Introduction
Herbal medicine has been widely employed to treat or prevent diseases using plant extracts. It is part of alternative medicine, which is rich in therapeutic phytochemicals that may lead to the development of novel drugs [1,2]. Olive leaves are one of the most common, traditional herbal teas used amongst Mediterranean people to cure certain conditions [3]. For this reason, interest in the potential health benefits of olive leaves has increased amongst scientists across various fields. The olive tree (Olea europaea L.) is one of the most important fruit trees in Mediterranean countries, where they cover 8 million ha, accounting for almost 98% of the world crop [3]. In Tunisia, olive agriculture is one of the most important agricultural activities [4]. Antioxidant, hypoglycemic, antihypertensive, antimicrobial, and anti-atherosclerotic effects of olive leaves have been reported in various studies [5,6,7,8,9]. The olive leaves from O. europaea are rich in biophenols, such as oleuropein (Ole), verbascoside, ligstroside, tyrosol, and hydroxytyrosol [10]. These compounds have shown several biological activities, including antioxidant, antithrombotic, as well as skin photoprotective properties [10,11]. Furthermore, some of these compounds have demonstrated antimicrobial activity by inhibiting the growth of a wide variety of bacteria and fungi [12,13]. The antibacterial effect of olive leaves has been correlated with the presence of olive phenolic compounds, such as tyrosol (TyEDA) and hydroxytyrosol (HyEDA) [14]. O. europaea is known to contain a mixture of polyphenolic compounds, including oleuropein, oleuropein aglycone, elenolic acid, and hydroxytyrosol, which are readily absorbed and bioavailable. The biological activities of O. europaea are mainly derived from these compounds [15]. Fredrickson [16] demonstrated that O. europea has potent antiviral activities against the herpes virus, hepatitis virus, rotavirus, bovine rhinovirus, canine parvovirus, and feline leukemia virus. Indeed, the antiviral effect of olive extracts from Iran has been demonstrated against herpes simplex virus type 1 (HSV-1) in Vero cells [17]. Herpes simplex virus represents a persistent human pathogen that resides in infected hosts for their lifetime [18]. Indeed, following primary infection, the HSV-1 virus can undergo lytic infection in epithelial cells and latent infection in sensory neurons [18]. Since HSV infections are often subclinical, the infection is widely becoming one of the world’s most prevalent sexually transmitted infections (STIs) [19]. The infection can be serious in immuno-compromised hosts, and may involve the central nervous system, which, if left untreated, could be associated with 70% of mortalities [20]. Acyclovir is widely used for the treatment of primary and recurrent HSV-1 infections [21]. Based on this evidence, the present study aimed to prepare and compare two olive leaf extracts from O. europea var. sativa (OESA) and O. europea var. sylvestris (OESY) from Sfax (south-east of Tunisia) in order to characterize their chemical composition and investigate their antibacterial and antiviral properties. The antimicrobial potential of OESA and OESY was evaluated against a range of Gram-positive and Gram-negative bacteria and the yeast Candida albicans. The antiviral mechanism exerted by OESA and OESY extracts was assessed against HSV-1.
2. Materials and Methods
2.1. Chemicals
Formic acid and methanol were LC-MS grade and purchased from Merck (Darmstadt, Germany). Reference standards oleuropein, rutin, luteolin-7-O-glucoside, and apigenin-7-O-rutinoside were purchased from Extrasynthese (Genay, France), whereas luteolin-7-O-rutinoside was purchased from Merck (Darmstadt, Germany). Other chemicals were of analytic grade.
2.2. Sample Origin
The samples of the olive leaves used were collected during the month of October 2018 from Sfax, southeast of Tunisia. Our choice was directed towards the most important olive variety Chemlali, Olea europea L. var. sativa (OESA) and Olea europea var. sylvestris (OESY).
2.3. Sample Preparation
OLSA and OLSY olive leaves were air-dried in the dark for three weeks, after which they were powdered by a mechanical grinder. The extraction was carried out using a mixture of water/ethanol (50:50, v/v) by simple maceration for 24 h with gentle stirring. Finally, the freeze-dried extracts were stored at 4 °C until further use.
2.4. Phytochemical Screening
2.4.1. Total Phenols
The total phenol content was determined according to Smeriglio et al. [22]. Briefly, 50 μL of OESA, OESY (0.5–4.0 mg/mL), and gallic acid as the reference standard (75.0–600 μg/mL) were added to the Folin-Ciocalteu reagent (1:10 v/v) and brought to 1 mL with deionized water. After 3 min, 10% sodium carbonate (500 mL) was added, and the sample was left in the dark at room temperature (RT) for 1 h and mixed every 10 min. The absorbance was recorded at 785 nm by an UV–Vis spectrophotometer (Shimadzu UV-1601, Kyoto, Japan). The results were expressed as g of gallic acid equivalents (GAE)/100 g of dry extract (DE).
2.4.2. Flavonoids
The flavonoid content was evaluated according to Smeriglio et al. [23]. Briefly, 200 μL of OESA, OESY (0.375–3.0 mg/mL), and rutin as the reference standard (0.125–1.0 mg/mL) were added to 2 mg/mL of AlCl3 (1:1, v/v) and brought to 1.6 mL with 50 mg/mL sodium acetate. After 2.5 h, the absorbance was recorded at 440 nm by an UV–Vis spectrophotometer (Shimadzu UV-1601, Kyoto, Japan). The results were expressed as g of quercetin equivalents (QE)/100 g DE.
2.5. LC-DAD-ESI-MS Analysis
The phytochemical analysis of OESA and OESY was carried out using an Agilent high-performance liquid chromatography system (HPLC 1100 series) equipped with an UV–Vis photodiode array (PDA-G1315) detector and an ion trap mass spectrometer detector (IT-6320). An electrospray ionization (ESI) source was used in full scan mode, monitoring the precursor ions between m/z 50 and m/z 1000 in negative polarity by using the following parameters: capillary voltage, 3.5 kV; drying gas temperature, 350 °C; nitrogen flow, 10 L/min; and nitrogen pressure, 50 psi. Data processing was carried out by Agilent 6300 Series Ion Trap LC/MS system software (version 6.2). The chromatographic separation was achieved by a Luna Omega PS C18 column (150 mm × 2.1 mm, 5 µm; Phenomenex, CA, USA) using solvent A (0.1% formic acid) and solvent B (methanol) as the mobile phase. The elution program was the following: 0–2 min, 5% B; 2–10 min, 25% B; 10–20 min, 40% B; 20–30 min, 50% B; 30–40 min, 100% B; 40–45 min, 5% B; and 45–60 min, 5% B. The flow rate was 0.3 mL/min, whereas the column temperature and the injection volume were 25 °C and 5 μL, respectively. UV–Vis spectra were recorded in the range of 190–700 nm, and chromatograms were acquired at 254, 280, 340, 370, and 520 nm. The acquisition wavelength chosen to show and compare the phytochemical profile of both extracts, at which all of the identified peaks were visible, was 254 nm. Peaks were identified by comparing the retention time, mass, and UV–Vis spectra with literature data and, when available, with reference standards (oleuropein, rutin, luteolin-7-O-glucoside, luteolin-7-O-rutinoside, apigenin-7-O-rutinoside).
2.6. Antimicrobial Assay
2.6.1. Microbial Strains and Culture Conditions
A range of strains obtained from the University of Messina’s in-house culture collection (Messina, Italy) was used for the susceptibility studies: Staphylococcus aureus ATCC 6538, methicillin-resistant S. aureus ATCC 43300 (MRSA), 12 clinical isolates of S. aureus obtained from the pharynges (strains 26, 526, 531, 550, 808, 814), from duodenal ulcers (strains 8, 14), from hip prostheses (strains 3, 6, 32, 84), Escherichia coli ATCC 10536, Pseudomonas aeruginosa ATCC 9027, and Candida albicans ATCC 10231. All bacterial strains were grown in Mueller–Hinton Broth (MHB, Oxoid, CM0405) at 37 °C (18–20 h), whereas C. albicans was cultured in RPMI 1640 at 30 °C (24 h).
2.6.2. Susceptibility Assays
The minimum inhibitory concentration (MIC), the minimum bactericidal concentration (MBC), and the minimum fungicidal concentration (MFC) of OESA and OESY were determined by the broth microdilution method, according to CLSI [24]. The tested concentrations ranged from 2000 to 1.9 μg/mL of either OESA or OESY dissolved in DMSO. The final concentration of DMSO did not exceed 1% in each sample. The MIC was defined as the lowest concentration that completely inhibited bacterial growth after 20 h. The MFC was defined as the lowest concentration that completely inhibited fungal growth after 48 h. The MBCs were determined by seeding 20 μL from clear MIC wells onto Mueller–Hinton agar (MHA, Oxoid) plates. The MBC was defined as the lowest extract concentration that killed 99.9% of the final inocula after 24 h incubation.
2.7. Antiviral Assay
2.7.1. Cell Lines and Viruses
VERO cell lines (American Type Culture Collection) were propagated in minimal essential medium (EMEM) and supplemented with 6% fetal bovine serum (FBS) (Lonza, Belgium) at 37 °C under 5% CO2. The prototype HSV-1 (F) strain was kindly provided by Dr. Bernard Roizman (University of Chicago, Chicago, IL, USA). The HSV-1 viral stocks were obtained from cell-free supernatant of Vero cells infected with HSV-1. The recombinant virus HSV-1-VP26GFP expressing a GFP-tagged VP26 protein was propagated in Vero cells as previously described [25].
2.7.2. Cell Proliferation Assay
The cell viability assay was performed as previously described [26]. Briefly, Vero cells were grown in 96-well plates and treated with different concentrations of OESA and OESY extracts (0.05 mg/mL, 0.1 mg/mL, 0.2 mg/mL, 0.4 mg/mL, 0.8 mg/mL, and 1 mg/mL) for 72 h. The cell viability was determined with a cytotoxicity bioassay kit (Lonza Group Ltd., Basel, Switzerland) according to the manufacturer’s instructions. The GloMax® Multi Microplate Luminometer (Promega Corporation, 2800 Woods Hollow Road, Madison, WI, USA) in combination with the ViaLight™ plus cell proliferation and cytotoxicity bioassay kit was used to detect the emitted light intensity related to ATP degradation. The measured luminescence value was converted into the cell proliferation index (%) as previously reported [19].
2.7.3. Plaque Reduction Assay
The antiviral activity was evaluated by plaque reduction assay. The Vero cells were seeded on 24-well plates and infected with the virus inoculum for 1 h at 37 °C with gentle shaking. The virus was diluted to yield 60 plaques/100 µL. A time-of-addition approach was used: (i) the virus inoculum was added on Vero cells and, after the incubation time, the monolayers were covered with a medium containing 0.8% methylcellulose in the presence of OESA and OESY extracts; and (ii) the virus inoculum was added on Vero cells pre-treated with OESA and OESY extracts and, after infection, the monolayers were covered with a medium containing 0.8% methylcellulose. The concentrations used for the antiviral assay were as follows: 0.1 mg/mL, 0.2 mg/mL, 0.4 mg/mL, 0.8 mg/mL, and 1 mg/mL. Acyclovir was included as the control at various concentrations (1, 10, and 20 µM). After three days, the cells were fixed, stained with crystal violet, and visualized with an inverted microscope (Leica DMIL, Nuloch, Germany) for plaque detection. After the incubation time, the inoculum was removed, and the monolayers were overlaid with Dulbecco’s Modified Eagle’s Medium containing 0.8% methylcellulose in the presence of the extracts. The plates were incubated at 37 °C with 5% CO2 for 72 h, and the plaques were visualized by staining the cells with crystal violet.
2.7.4. The Binding Assay
Vero cells (4 × 105 cells/well) were cultured in 12-well plates and uninfected (mock) or infected with HSV-1 VP26GFP virus at a multiplicity of infection (MOI) of 0.5 PFU/cell. The infection was carried out by pre-treating the cells and the viral suspension with 0.1 mg/mL of OESA and 0.2 mg/mL of OESY, respectively, for 1 h. After the incubation time, the virus was adsorbed on pre-treated cells for 1 h at 4 °C. The infection was carried out at 4 °C to allow only the binding of the virus, but not the entry into the cells during the infection step. The virus inoculum was then removed, and the monolayers were incubated with a medium containing the extracts. Medium alone or containing DMSO were used as the controls.
2.8. Western Blot Analysis and Antibodies
Cellular proteins were extracted from Vero cells using SDS sample buffer 1× (62.5 mM of Tris-HCl, pH = 6.8; Dithiothreitol (DTT) 1 M; 10% glycerol; 2% SDS; 0.01% Bromophenol Blue), and immunoblot analysis was performed using an equal quantity of proteins. The proteins were revolved on SDS 10% polyacrylamide gel electrophoresis (PAGE) and transferred to nitrocellulose membranes (BioRad Life Science Research, Hercules, CA, USA). The membranes were probed overnight at 4 °C with specific antibodies to detect GFP-VP26 protein. Specific proteins were detected with a secondary anti-mouse antibody linked to horseradish peroxidase (HRP). GAPDH was used as a loading control. The chemiluminescence was detected by using Western HRP substrate (Merk, Millipore, Burlington, MA, USA). Immunoblot band intensity was quantified by densitometry analysis using the TINA software (version 2.10, Raytest, Straubenhardt, Germany). Anti-GFP (sc-9996) and Anti-GAPDH (sc-32233) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, United States). Goat anti-mouse immunoglobulin G (IgG) antibody-HRP conjugate was purchased from Merk, Millipore.
2.9. Viral DNA Extraction and Real-Time PCR Analysis
Cellular DNA was extracted from TRIzol-lysed cells according to the manufacturer’s instructions (Life Technologies, Carlsbad, CA, USA). Briefly, the DNA solution was precipitated from the interphase and organic phase with 100% ethanol. The DNA pellet was washed twice with 0.1 M of sodium citrate in 10% ethanol and dissolved in 8 mM of NaOH. Real-time PCR analysis was performed by using a specific TaqMan HSV-1 probe in a Cepheid SmartCycler II System (Cepheid Europe, Maurens-Scopont, France); 1 µg of DNA template was mixed with 1 µM of deoxyribonucleotide triphosphate (dNTP) mix, 0.5 μM of forward and reverse primers, 1 µM of TaqMan probe, 1× NH4 reaction buffer, 2 mM of MgCl2, and 5 U/µL of DNA polymerase BIOTAQ (BIO-21040 Bioline) in a total volume of 25 µL. The oligonucleotide primers used were: HSV-1 Fw 5′-catcaccgacccggagagggac; HSV-1 Rev 5′gggccaggcgcttgttggtgta, HSV-1 TaqMan probe 5′-6FAM-ccgccgaactgagcagacacccgcgc-TAMRA, (6FAM is 6-carboxyfluorescein and TAMRA is 6-carboxytetramethylrhodamine). The amplification was performed as follows: (i) 10 min at 95 °C, (ii) 30 s at 95 °C for 40 cycles, (iii) 30 s at 55 °C, (iv) 30 s at 72 °C, and (v) 5 min at 72 °C. A negative sample was used as the amplification control for each run. The quantitation of HSV-1 DNA was generated using GAPDH as a housekeeping gene with the comparative Ct method. DNA extracted from Vero cells infected and treated with Acyclovir (20 µM) was used as a positive control.
2.10. Statistical Analysis
The statistical analysis was performed with GraphPad Prism 8 software (GraphPad Software, San Diego, CA, USA) by using one-way analysis of variance (ANOVA). The significance of the p-value was indicated with asterisks (*, **, ***, ****), which indicate the significance of the p-value less than 0.05, 0.01, 0.001, 0.0001 respectively. The half maximal cytotoxic concentration (CC50) and the half maximal effective concentration (EC50) values were calculated by using non-linear regression analysis.
3. Results
3.1. Phytochemical Analyses
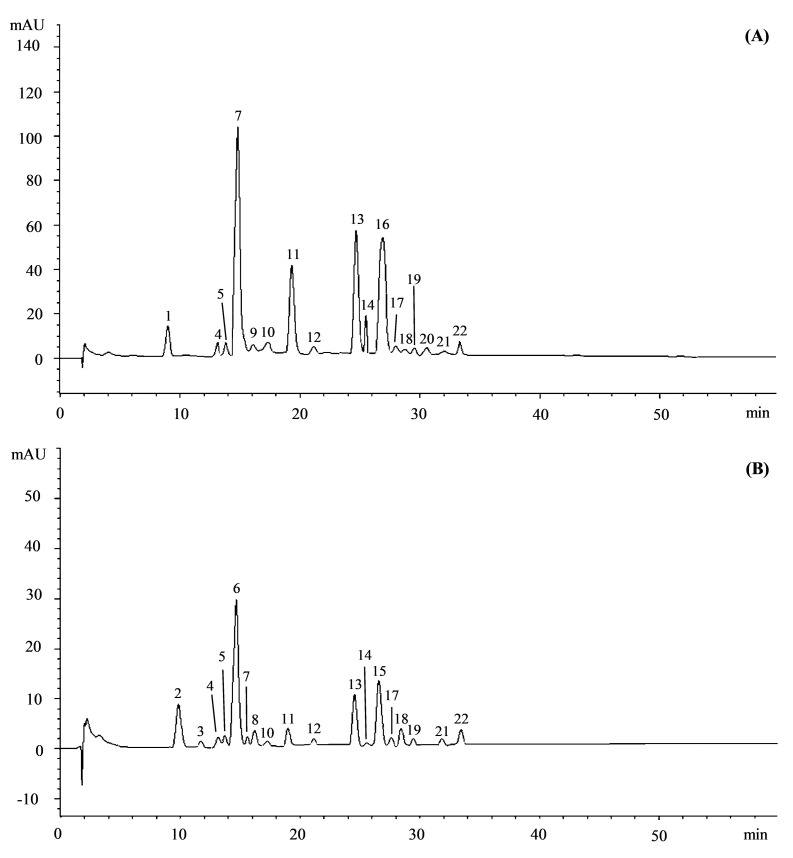
Preliminary phytochemical screening showed that the leaf hydroalcoholic extract of O. europea var. sativa (OESA) showed a higher content of total phenols (5.04 ± 0.29 g GAE/100 g DE vs. 4.40 ± 0.38 g GAE/100 g DE) and flavonoids (35.25 ± 3.19 g QE/100 g DE vs. 31.10 ± 1.81 g QE/100 g DE) with respect to the leaf hydroalcoholic extract of O. europea var. sylvestris (OESY). Comparative RP-LC-DAD-ESI-MS analysis of OESA and OESY extracts (Figure 1) revealed the presence of many polyphenols, characteristic of olive leaves.
Figure 1.
Representative LC-DAD chromatograms of O. europea L. var. sativa (OESA, panel A) and O. europea var. sylvestris (OESY, panel B) leaf hydroalcoholic extracts acquired at 254 nm. Peak numbers refer to the compounds listed in Table 1.
Seventeen and eighteen compounds were identified in the OESA and OESY leaf extracts, respectively. Table 1 shows the phytochemical profile of both extracts by listing the compounds according to their elution order.
Table 1.
Phytochemical profile of the leaf hydroalcoholic extract of O. europea L. var. sativa (OESA) and O. europea var. sylvestris (OESY) by LC-DAD-ESI-MS analysis.
| Peak n. | Compound | RT 1 | λmax | [M-H]− | MW 2 | Area % 3 | |
|---|---|---|---|---|---|---|---|
| OESA | OESY | ||||||
| 1 | Dihydroxybenzoic acid hexoside pentoside | 9.083 | 296 | 447 | 448 | 4.95 | - |
| 2 | Valoneic acid dilactone | 9.9 | 256;365 | 469 | 470 | - | 8.69 |
| 3 | Dicaffeoylquinic acid | 11.7 | 218;327 | 515 | 516 | - | 5.87 |
| 4 | Gallagic acid | 13.309 | 234;296 | 603 | 604 | 0.97 | 10.38 |
| 5 | Diellagilactone | 13.831 | 252;377 | 601 | 602 | 1.00 | 0.89 |
| 6 | Decaffeoylverbascoside | 14.886 | 236;280 | 461 | 462 | - | 23.07 |
| 7 | Oleoside/Secologanoside | 14.955 | 244 | 389 | 390 | 32.68 | 0.94 |
| 8 | Epicatechin 3-p-hydroxybenzoate | 16.199 | 282;318 | 409 | 410 | - | 2.55 |
| 9 | Elenoic acid hexoside | 16.230 | 238 | 403 | 404 | 0.73 | - |
| 10 | Hydroxyoleuropein isomer I | 17.431 | 232;282 | 555 | 556 | 1.06 | 2.94 |
| 11 | Oleanolic acid | 19.461 | 232 | 454 | 455 | 11.48 | 1.91 |
| 12 | Hydroxyoleuropein isomer II | 21.390 | 232;282 | 555 | 556 | 0.58 | 3.75 |
| 13 | Luteolin-7-O-rutinoside | 24.821 | 258;344 | 593 | 594 | 13.86 | 9.84 |
| 14 | Rutin | 25.560 | 256;358 | 609 | 610 | 1.99 | 0.80 |
| 15 | Oleuropein hexoside | 26.855 | 232;282 | 701 | 702 | - | 11.07 |
| 16 | Oleuropein | 26.950 | 232;286 | 539 | 540 | 18.03 | - |
| 17 | Apigenin-7-O-rutinoside | 27.421 | 252;336 | 577 | 578 | 1.90 | 2.26 |
| 18 | Luteolin-7-O-glucoside | 28.326 | 268;342 | 447 | 448 | 6.14 | 6.92 |
| 19 | Hydroxyphloretin 2′-O-xylosylglucoside | 29.455 | 250;340 | 583 | 584 | 0.74 | 1.29 |
| 20 | Vitisin A | 30.440 | 388;510 | 560 | 561 | 0.76 | - |
| 21 | β-Sitosteryl ferulate | 32.140 | 240;294;318 | 590 | 591 | 0.84 | 0.56 |
| 22 | Lucidumoside C | 33.500 | 240;284 | 583 | 584 | 2.30 | 6.27 |
1 RT, Retention time; 2 MW, Molecular weight; 3 Results were expressed as mean area percentage of three independent experiments (n = 3) and is calculated with respect to the total peaks found.
The numbers refer to the peaks shown in Figure 1. Secoiridoids represented the most abundant class of compounds identified in OESA (55.38%), followed by flavones (21.90%), terpenoids (11.48%), and phenolic acids (4.95%). Minor compounds identified were other flavonoids (2.73%), ellagitannins (1.97%), phytosterols (0.84%), and stilbenoids (0.76%). On the contrary, in OESY, the content of secoiridoids (24.97%) was almost comparable to the content of phenylethanoids (23.07%), followed by flavones (19.02%), ellagitannins (11.27%), hydrolysable tannin (8.69%), and phenolic acids (5.87%). Minor compounds identified were other flavonoids (4.64%), terpenoids (1.91%), and phytosterols (0.56%). Bold numbers in Table 1 refer to the most abundant compounds identified in both leaf hydroalcoholic extracts. Oleoside/secologanoside (32.68%) was the most abundant compound identified in the OESA, followed by oleuropein (18.03%), luteolin-7-O-rutinoside (13.86%), oleanolic acid (11.48%), and luteolin-7-O-glucoside (6.14%). On the contrary, decaffeoylverbascoside (23.07%) was the most abundant compound identified in OESY, followed by oleuropein hexoside (11.07%), gallagic acid (10.38%), luteolin-7-O-rutinoside (9.84%), and valoneic acid dilactone (8.69%).
3.2. Antimicrobial Potential
The MIC values of OESA and OESY against the tested strains are shown in Table 2 and Table 3. Both extracts were active against the Gram-positive ATCC strains included in the study (MIC values between 7.81 and 15.62 μg/mL and between 15.62 and 31.25 μg/mL for OESY and OESA, respectively), whereas no activity was found against the Gram-negative bacteria or the yeast. As expected, the MIC values were lower for S. aureus ATCC 6538, demonstrating the higher sensitivity of this strain compared to the MRSA strain. The activity was always bacteriostatic rather than bactericidal. Amongst the clinical isolates, two strains were sensitive to both extracts, OESY being more active than OESA. The clinical isolates used in this study were previously characterized in relation to their antibiotic resistance [27]. Interestingly, strain 808 was sensitive to all antibiotics tested, with the exception of benzyl penicillin, whereas strain 6 was resistant to oxacillin, but sensitive to cefoxitin. As expected, the bacteriostatic inhibition of the clinical isolates was achieved at a higher concentration of both extracts compared with the standard strains.
Table 2.
MICs of OESY and OESA (expressed as µg/mL) against Gram-positive bacteria, Gram-negative bacteria, and the yeast C. albicans.
| Strain | OESY | OESA |
|---|---|---|
| E. coli ATCC 10536 | NA | NA |
| S. aureus ATCC 6538 | 7.81–15.62 | 15.62–31.25 |
| S. aureus ATCC 43300 | 500–1000 | 1000 |
| P. aeruginosa ATCC 9027 | NA | NA |
| C. albicans ATCC 10231 | NA | NA |
MICs, minimal inhibitory concentrations; OESY, extract of O. europea var. sylvestris; OESA, extract of O. europea var. sativa. NA, not active.
Table 3.
MICs of OESY and OESA (expressed as µg/mL) against clinical isolates of S. aureus.
| Strain | OESY | OESA |
|---|---|---|
| S. aureus strain 3 | NA | NA |
| S. aureus strain 6 | 1000 | 2000 |
| S. aureus strain 8 | NA | NA |
| S. aureus strain 14 | NA | NA |
| S. aureus strain 26 | NA | NA |
| S. aureus strain 32 | NA | NA |
| S. aureus strain 84 | NA | NA |
| S. aureus strain 526 | NA | NA |
| S. aureus strain 531 | NA | NA |
| S. aureus strain 550 | NA | NA |
| S. aureus strain 808 | 1000 | 2000 |
| S. aureus strain 814 | NA | NA |
MICs, minimal inhibitory concentrations; OESY, extract of O. europea var. sylvestris; OESA, extract of O. europea var. sativa. NA, not active.
The comparison between the two extracts showed a greater antimicrobial potential exerted by OESY rather than OESA, reflecting the different phytochemical composition of the two extracts (Table 1). This trend did not seem to relate to the total phenol content, which was higher in OESA. However, the phytochemical profile of the two extracts may be responsible for the different antimicrobial effect: secoiridoids were the most abundant class of compounds in OESA (55.38%), followed by flavones (21.90%) and terpenoids (11.48%), whereas the content of secoiridoids (24.97%) was almost comparable to phenylethanoids (23.07%) in OESY, followed by flavones (19.02%) and ellagitannins (11.27%).
3.3. Cytotoxicity of Olive Leaf Extracts on Cell Cultures
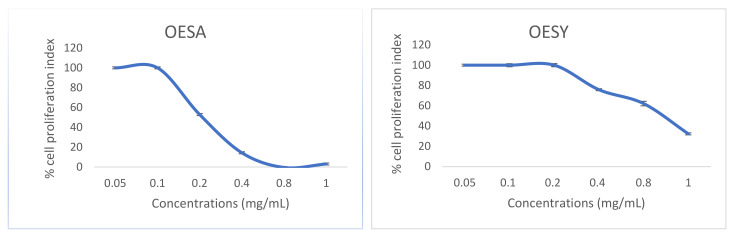
In order to examine the cytotoxicity effect of olive leaf extracts on Vero cells, we incubated the cells in the presence of different concentrations of OESA and OESY extracts for 72 h. Samples were then collected, and the quantification of the emitted light intensity related to ATP degradation was measured. Based on these results, the CC50 values were 0.2 mg/mL and 0.82 mg/mL for OESA and OESY, respectively (Figure 2).
Figure 2.
Viability assay in Vero cells treated with olive leaf extracts OESA and OESY. Vero cells were treated with different concentrations of olive leaf extracts (0.05 mg/mL, 0.1 mg/mL, 0.2 mg/mL, 0.4 mg/mL, 0.8 mg/mL, 1 mg/mL). The cells were collected 72 h post-treatment, and the luminescence value was converted into the cell proliferation index (%) as described in the Materials and Methods section. The assay was performed as the means of triplicates ± SD. OESY, extract of O. europea var. sylvestris; OESA, extract of O. europea var. sativa.
3.4. Antiviral Activity of OESA and OESY
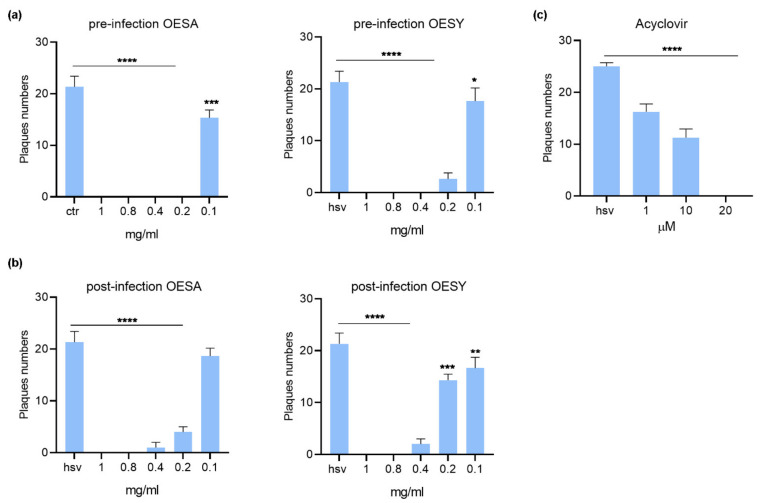
The antiviral effect of OESA and OESY was determined against herpes simplex virus type 1 (HSV-1) using the plaque reduction assay. A time-of-addition assay was used. For the pre-infection treatment assay, Vero cells were treated with the extracts for 1 h and then infected with HSV-1 and incubated for 1 h at 37 °C. After the incubation time, the inoculum was removed, and the monolayers were overlaid with a medium containing 0.8% methylcellulose. For the post-infection treatment assay, Vero cells were infected with HSV-1 and incubated for 1 h at 37 °C. After the incubation time, the inoculum was removed and the monolayers were overlaid with a medium containing 0.8% methylcellulose in the presence of ethanolic extract of the leaf of OESA and OESY. The plates were incubated at 37 °C and 5% CO2 for 72 h, and the plaques were visualized by staining cells with crystal violet. Acyclovir was used as a control. The results showed a dose-dependent antiviral activity for both OESA and OESY (Figure 3). However, as indicated in Table 3, the selectivity index (SI) was higher for OESY (SI between 4.1 and 7.4) compared to OESA (SI between 1.3 and 1.6), and the pre-infection treatment condition was found to be more effective compared to the post-infection treatment for both types of extracts (Table 4).
Figure 3.
Plaque reduction assay from time-of-addition assay: (a) pre-infection condition, (b) post-infection condition treatments, and (c) Acyclovir control. Results are the mean ± SD of triplicate experiments, and asterisks indicate a significant p-value (* p < 0.05, ** p < 0.01, *** p < 0.001 and **** p < 0.0001). OESY, extract of O. europea var. sylvestris; OESA, extract of O. europea var. sativa.
Table 4.
Selectivity index (SI), cytotoxicity (CC50), and antiviral activity (EC50) of OESA and OESY leaf extracts.
| Extract | CC50 (mg/mL) | EC50 (mg/mL) | SI |
|---|---|---|---|
| OESA | |||
| pre-infection | 0.2 | 0.12 | 1.6 |
| post-infection | 0.2 | 0.15 | 1.3 |
| OESY | |||
| pre-infection | 0.82 | 0.11 | 7.4 |
| post-infection | 0.82 | 0.2 | 4.1 |
CC50: half maximal cytotoxic concentration; EC50: half maximal effective concentration; SI: ratio of EC50/CC50; OESY, extract of O. europea var. sylvestris; OESA, extract of O. europea var. sativa.
3.5. OESA and OESY Prevent the Binding of HSV-1 on Vero Cells
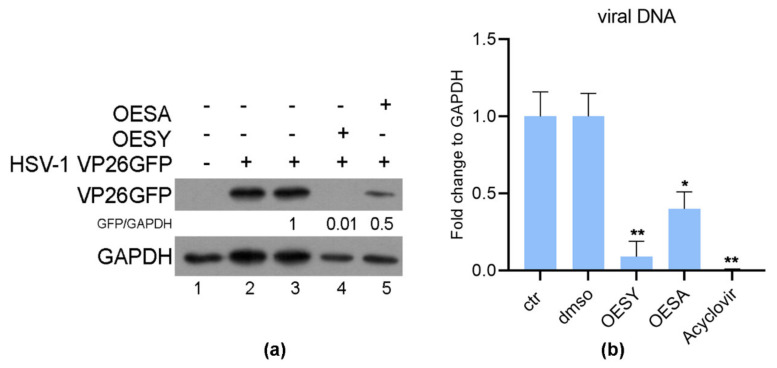
The results obtained from the plaque reduction assay indicated that the SI was higher when the cells were pre-treated with the extracts before infection. To confirm the hypothesis that the binding of the virus could be prevented by both extracts, a binding inhibition assay was performed as indicated in the Materials and Methods section. The binding inhibition assay was carried out by using a recombinant HSV-1-VP26GFP virus. The infection was carried out at 4 °C to allow the binding of the virus to the cell receptors, but not the entry. Twenty-four hours post-infection, the expression of the VP26GFP protein was detected by western blot analysis, and quantitative real-time PCR was carried out to quantify the viral DNA. DNA extracted from Vero cells infected and treated with Acyclovir (20 µM) was used as a positive control for the quantization of viral DNA. As shown in Figure 4, pretreatment of cells and the virus with either OESA (0.1 mg/mL) or OESY (0.2 mg/mL) was able to reduce the accumulation of VP26 viral protein, as well as the accumulation of viral DNA, compared to the DMSO control. Moreover, OESY showed a greater antiviral activity compared to OESA, confirming the previous results obtained in Figure 3.
Figure 4.
OESA and OESY prevent the binding of HSV-1. Vero cells infected or uninfected with HSV-1 VP26GFP, as described in the Materials and Methods section. Samples were processed 24 h p.i. for western blot analysis (a) and real-time PCR (b). DNA extracted from Vero cells infected and treated with Acyclovir (20 µM) was used as a positive control for the quantization of viral DNA. Data are expressed as the mean (± SD) of at least three experiments, and asterisks (* and **) indicate the significance of p-values less than 0.05 and 0.01, respectively. OESY, extract of O. europea var. sylvestris; OESA, extract of O. europea var. sativa.
4. Discussion
Plant extracts and pure compounds have been widely tested for their antioxidant and antimicrobial activity. Olive leaves have very good biological activities, such as antihypertensive, anti-atherogenic, anti-inflammatory, hypoglycemic, and hypocholesterolemic effects [28,29]. In the present study, the RP-LC-DAD-ESI-MS analysis revealed the presence of many polyphenols in OESA and OESY extracts. The obtained results indicated luteolin- and apigenin-7-O-glycosides to be the predominant flavonoids in olive leaves, followed by rutin. These data are in accordance with previous observations carried out in olive leaf extracts obtained from five cultivars grown in southern Spain [10], as well as Portuguese olive cultivars [30]. Moreover, Pereira and coworkers [3] identified rutin, apigenin-7-O-glucoside, and luteolin-7-O-glucoside as the most abundant flavonoids in an aqueous extract of olive leaves from northeast Portugal. These results were recently corroborated by Makowska-Wąs and coworkers [31], who identified luteolin-7-O-glucoside, rutin, and apigenin-7-O-glucoside as the predominant flavonoids in wild olive leaves harvested in South Portugal. Regarding secoiridoids, oleuropein generally represents the most characteristic oleoside derivative in olive leaves. However, its content and bioconversion occur in different districts of the olive tree according to different factors, such as plant maturation, cultivar type, and harvest time [32]. Indeed, as observed in Table 1, although only OESA showed the presence of oleuropein (18.03%), OESY contained oleuropein hexoside (11.07%), as well as the highest content of hydroxyoleuropein isomer I and II (2.94% and 3.75% vs. 1.06% and 0.58% in OESA). On the contrary, OESY showed the highest content of tannins (19.96% vs. 1.97% in OESA), and it was a rich source of decaffeoylverbascoside (23.07%), absent in OESA. This phenyleptanoid is a derivative of verbascoside, a typical hydroxycinnamic derivative of olive fruit, generally only found in small amounts in olive leaves [31]. However, the amount of this metabolite in olive leaves may significantly vary depending on pedo-climatic conditions [32]. Furthermore, it seems that a reverse relationship exists between the oleuropein and verbascoside content [32], which could explain the absence of oleuropein in OESY. However, beyond the different varieties, it is well-known that biotic and abiotic stressors play a pivotal role in influencing the polyphenolic content of olive leaves, as well as the relative quantitative distribution of the main metabolites [33]. LC-DAD-ESI-MS analysis of OESA and OESY extracts identified different compounds that have an antimicrobial effect justified in the present study. The hydroalcoholic extracts from O. europea leaves were effective against S. aureus strains. As often reported with plant-derived extracts [34,35], the present study confirmed that Gram-positive strains were more susceptible compared with Gram-negative bacteria. Amongst the Gram-positive human pathogens, S. aureus and methicillin-resistant S. aureus (MRSA) play a crucial role, being responsible for several infections, including skin, respiratory, and bone joint infection, as well as endocarditis, bacteremia, and toxic shock syndrome [36]. Due to the increased spread of multi-drug resistance, more effort has been focused on novel antimicrobial agents against S. aureus and MRSA. According to many reports, phenolic compounds isolated from olive leaves have substantial antimicrobial activity [5,37,38]. The effectiveness of certain phenolic compounds in olive leaves, including caffeic acid, oleuropein, rutin, and verbascoside against S. aureus, has been reported [3,39]. Therefore, we hypothesize that these compounds, as well as potential synergistic effects within the extracts, were responsible for the activity reported here against S. aureus. The high relative proportion of decaffeoylverbascoside, a caffeoyl phenylethanoid glycoside in OESY, may be related to the stronger antimicrobial effect against S. aureus compared to OESA. Besides, olive leaf extract had significant antiviral activity against HSV-1 [17]. It has also been reported that olive leaf extracts exhibit antiviral activities against human immunodeficiency virus type 1 (HIV-1) [40,41]. Olea europea exhibits antiviral activity against viral hemorrhagic septicemia rhabdovirus (VHSV) [42], and is known to contain a mixture of polyphenolic compounds, including oleuropein, oleuropein aglycone, elenolic acid, and hydroxytyrosol [43]. Oleuropein possesses well-documented antiviral activity [17]; its efficacy against hemorrhagic septicemia rhabdovirus (VHSV), hepatitis B virus (HBV), and human immunodeficiency virus (HIV) was demonstrated [44]. The beneficial effect of oleuropein against VHSV is exerted through a virucidal effect, reducing virus infectivity and avoiding cell-to-cell fusion of uninfected cells [45]. Alternatively, the viral particle integrity could be damaged, as has been previously observed for the effect of flavonones on HSV-1 [46]. The data presented here indicate that both OESA and OESY exert a dose-dependent, broad-spectrum antiviral activity against HSV-1. However, the selectivity index (SI) was higher for OESY (SI between 4.1 and 7.4) compared to OESA (SI between 1.3 and 1.6). It is worth noting that, in addition to their anti-oxidative and anti-inflammatory characteristics, richness in polyphenols in OESA and OESY extracts can be the basis for anti-infective properties. Several extracts were proposed to interfere with HSV-1 attachment to cells via its surface structures and proteins, thereby blocking its adsorption and penetration into host cells.
HSV-1 entry is essential for the initiation, spread, and maintenance of viral infection, and represents the target for antiviral mechanisms exerted by OESA and OESY extracts. Indeed, our studies indicated that the time-of-addition assay and the inhibition entry assay were essential for the antiviral effect (Figure 4). Overall, our findings demonstrate that OESA and OESY interfere with the virus attachment to cell receptors, and thus reduce HSV-1 entry and replication on Vero cells.
5. Conclusions
In conclusion, the current study revealed that polyphenols from O. europea leaf extract exhibit an antiviral effect against HSV-1 and antimicrobial activity against S. aureus, which was stronger for the methicillin sensitive strains. Our findings demonstrated that O. europea leaf extracts, after one hour of viral adsorption, resulted in a significant neutralization of the virus in Vero cells. It is possible to hypothesize that one potential mechanism for this inhibitory effect of O. europea leaf extracts on the HSV-1 lytic cycle is the blocking of virion entry into the cells. However, there are still numerous questions that need to be answered, which will require further research. In particular, further studies are needed to explore the molecular mechanisms responsible for the antiviral activity, as well as the roles of polyphenols from O. europea leaves that may serve as a source of novel pharmacological treatment. Lastly, OESA and OESY extracts could be used in combination with other antimicrobial and antiviral drugs to reduce the emergence of resistant strains.
This study suggests new strategies for manipulating HSV-1 infection both for prevention and therapeutic purposes.
Author Contributions
I.B.-A. and M.M.-P. equally contributed; I.B.-A. and M.M.-P. performed the experiments on antiviral activity and viability assay; A.S. and D.T. performed the phytochemical screening and the chemical characterization; R.P. performed the statistical analysis; M.D. performed the experiments on antimicrobial activity. B.G. and H.A. contributed to the extraction and the concept; Writing—review & editing, M.T.S. and G.M. Funding acquisition, M.T.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the University of Messina Research & Mobility 2016 Project (Project Code: RES_AND_MOB_2016_Sciortino) and the University of Messina FFABR 2019 Project.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Volker S., Rudolf H., Tyler V. Medicinal Plants, Phytomedicines, and Phytotherapy. Ration. Phytother. 2004 doi: 10.1007/978-3-662-09666-6_1. [DOI] [Google Scholar]
- 2.Babulka P. Plantes médicinales du traitement des pathologies rhumatismales: De la médecine traditionnelle à la phytothérapie moderne. Phytotherapie. 2007;5:137–145. doi: 10.1007/s10298-007-0240-8. [DOI] [Google Scholar]
- 3.Pereira A., Ferreira I., Marcelino F., Valentao P., Andrade P.B., Seabra R., Estevinho L., Bento A. Phenolic compounds and antimicrobial activity of olive (Olea europaea L. Cv. Cobranc, osa) leaves. Molecules. 2007;12:1153–1162. doi: 10.3390/12051153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bouaziz M., Chamkha M., Sayadi S. Comparative study on phenolic content and antioxidant activity during maturation of the olive cultivar Chemlali from Tunisia. J. Agric. Food Chem. 2005;52:5476–5481. doi: 10.1021/jf0497004. [DOI] [PubMed] [Google Scholar]
- 5.Sedef N.E., Sibel K. Olive tree (Olea europaea) leaves: Potential beneficial effects on human health. Nutr. Rev. 2009;67:632–638. doi: 10.1111/j.1753-4887.2009.00248.x. [DOI] [PubMed] [Google Scholar]
- 6.Borjan D., Leitgeb M., Knez Ž., Hrnčič M.K. Microbiological and Antioxidant Activity of Phenolic Compounds in Olive Leaf Extract. Molecules. 2020;25:5946. doi: 10.3390/molecules25245946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lockyer S., Rowland I., Spencer J.P.E., Yaqoob P., Stonehouse W. Impact of phenolic-rich olive leaf extract on blood pressure, plasma lipids and inflammatory markers: A randomised controlled trial. Eur. J. Nutr. 2016;56:1421–1432. doi: 10.1007/s00394-016-1188-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zorić N., Kopjar N., Kraljić K., Oršolić N., Tomić S., Kosalec I. Olive leaf extract activity against Candida albicans and C. dubliniensis—The in vitro viability study. Acta Pharm. 2016;66:411–421. doi: 10.1515/acph-2016-0033. [DOI] [PubMed] [Google Scholar]
- 9.Liu Y., McKeever L.C., Malik N.S.A. Assessment of the Antimicrobial Activity of Olive Leaf Extract Against Foodborne Bacterial Pathogens. Front. Microbiol. 2017;8:113. doi: 10.3389/fmicb.2017.00113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benavente-Garcıa O., Castillo J., Lorente J., Ortuno A., Del Rıo J.A. Antioxidant activity of phenolics extracted from Olea europaea L. leaves. Food Chem. 2000;68:457–462. doi: 10.1016/S0308-8146(99)00221-6. [DOI] [Google Scholar]
- 11.Saija A., Uccella N. Olive biophenols: Functional effects on human wellbeing. TIFS. 2001;11:357–363. doi: 10.1016/S0924-2244(00)00068-6. [DOI] [Google Scholar]
- 12.Hirschman S.Z. Inactivation of DNA polymerases of murine leukaemia viruses by calcium elenolate. Nat. N. Biol. 1972;238:277–279. doi: 10.1038/newbio238277a0. [DOI] [PubMed] [Google Scholar]
- 13.Bisignano G., Tomaino A., Lo Cascio R., Crisafi G., Uccella N., Saija A. On the in-vitro antimicrobial activity of oleuropein and hydroxytyrosol. J. Pharm. Pharmacol. 1999;51:971–974. doi: 10.1211/0022357991773258. [DOI] [PubMed] [Google Scholar]
- 14.Medina E., De Castro A., Romero C., Ramírez E., Brenes M. Effect of antimicrobial compounds from olive products on microorganisms related to health, food and agriculture. Microb. Pathog. Strateg. Combat. Sci. Technol. Educ. 2013;2:1087–1094. [Google Scholar]
- 15.Visiol F., Galli C. Antioxidant and otheir biological activities of phenols from olives and Olive oil. Med. Res. Rev. 2002;22:65–75. doi: 10.1002/med.1028. [DOI] [PubMed] [Google Scholar]
- 16.Fredrickson W.R. Method and Composition for Antiviral Therapy with Olive Leaves. No. 6,117,844. U.S. Patent. 2000
- 17.Motamedifar M., Nekooeian A.A., Moatari A. The Effect of Hydroalcoholic Extract of Olive Leaves against Herpes Simplex Virus Type 1. Iran. J. Med. Sci. 2007;32:222–227. [Google Scholar]
- 18.Arvin A., Campadelli-Fiume G., Mocarski E., Moore P.S., Roizman B., Whitley R., Yamanishi K. Human Herpesviruses Biology, Therapy, and Immunoprophylaxis. Cambridge University Press; Cambridge, UK: 2007. pp. 1–1408. [PubMed] [Google Scholar]
- 19.Bisignano C., Mandalari G., Smeriglio A., Trombetta D., Pizzo M.M., Pennisi R., Sciortino M.T. Almond Skin Extracts Abrogate HSV-1 Replication by Blocking Virus Binding to the Cell. Viruses. 2017;9:178. doi: 10.3390/v9070178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tyler K.L. Herpes simplex virus infections of the central nervous system: Encephalitis and meningitis, including Mollaret’s. Herpes. 2004;11(Suppl. 2):57A–64A. [PubMed] [Google Scholar]
- 21.Sarisky R.T., Crosson P., Cano R., Quail M.R., Nguyen T.T., Wittrock R.J., Bacon T.H., Sacks S.L., Caspers-Velu L., Hodinka R.L., et al. Comparison of methods for identifying resistant herpes simplex virus and measuring antiviral susceptibility. J. Clin. Virol. 2002;23:191–200. doi: 10.1016/S1386-6532(01)00221-9. [DOI] [PubMed] [Google Scholar]
- 22.Smeriglio A., Mandalari G., Bisignano C., Filocamo A., Barreca D., Bellocco E., Trombetta D. Polyphenolic content and biological properties of Avola almond (Prunus dulcis Mill. D.A. Webb) skin and its industrial byproducts. Ind. Crop. Prod. 2016;83:283–293. doi: 10.1016/j.indcrop.2015.11.089. [DOI] [Google Scholar]
- 23.Smeriglio A., Denaro M., Barreca D., D’Angelo V., Germanò M.P., Trombetta D. Polyphenolic profile and biological activities of black carrot crude extract (Daucus carota L. ssp. sativus var. atrorubens Alef.) Fitoterapia. 2018;124:49–57. doi: 10.1016/j.fitote.2017.10.006. [DOI] [PubMed] [Google Scholar]
- 24.CLSI . M100-S22: Institute Performance Standards for Antimicrobial Susceptibility Testing. Clinical and Laboratory Standards Institute (CLSI); Wayne, PA, USA: 2012. Twentieth Informational Supplement. [Google Scholar]
- 25.Siracusano G., Venuti A., Lombardo D., Mastino A., Esclatine A., Sciortino M.T. Early activation of MyD88-mediated autophagy sustains HSV-1 replication in human monocytic THP-1 cells. Sci. Rep. 2016;6:31302. doi: 10.1038/srep31302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Musarra-Pizzo M., Pennisi R., Ben-Amor I., Smeriglio A., Mandalari G., Sciortino M.T. In Vitro Anti-HSV-1 Activity of Polyphenol-Rich Extracts and Pure Polyphenol Compounds Derived from Pistachios Kernels (Pistacia vera L.) Plants. 2020;9:267. doi: 10.3390/plants9020267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bisignano C., Ginestra G., Smeriglio A., La Camera E., Crisafi G., Franchina F.A., Tranchida P.Q., Alibrandi A., Trombetta D., Mondello L., et al. Study of the Lipid Profile of ATCC and Clinical Strains of Staphylococcus aureus in Relation to Their Antibiotic Resistance. Molecules. 2019;24:1276. doi: 10.3390/molecules24071276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Briante R., Patumi M., Terenziani S., Bismuto E., Febbraio F., Nucci R. Olea europaea L. leaf extract and derivatives: Antioxidant properties. J. Agric. Food Chem. 2002;50:4934–4940. doi: 10.1021/jf025540p. [DOI] [PubMed] [Google Scholar]
- 29.Romero M., Toral M., Gomez-Guzman M., Jimenez R., Galindo P., Sanchez M., Olivares M., Galvez J., Duarte J. Antihypertensive effects of oleuropeinenriched olive leaf extract in spontaneously hypertensive rats. Food Funct. 2016;7:584–593. doi: 10.1039/C5FO01101A. [DOI] [PubMed] [Google Scholar]
- 30.Meirinhos J., Silva B.M., Valentão P., Seabra R.M., Pereira J.A., Dias A., Andrade P.B., Ferreres F. Analysis and quantification of flavonoidic compounds from Portuguese olive (Olea europaea L.) leaf cultivars. Nat. Prod. Res. 2005;19:189–195. doi: 10.1080/14786410410001704886. [DOI] [PubMed] [Google Scholar]
- 31.Makowska-Wąs J., Galanty A., Gdula-Argasińska J., Tyszka-Czochara M., Szewczyk A., Nunes R., Carvalho I.S., Michalik M., Paśko P. Identification of Predominant Phytochemical Compounds and Cytotoxic Activity of Wild Olive Leaves (Olea europaea L. ssp. sylvestris) Harvested in South Portugal. Chem. Biodivers. 2017;14:e1600331. doi: 10.1002/cbdv.201600331. [DOI] [PubMed] [Google Scholar]
- 32.Termentzi A., Halabalaki M., Skaltsounis A.L. Olive and Olive Oil Bioactive Constituents. AOCS Press; Cambridge, MA, USA: 2015. From Drupes to Olive Oil: An Exploration of Olive Key Metabolites; pp. 147–177. [DOI] [Google Scholar]
- 33.Talhaoui N., Taamalli A., Gómez-Caravaca A.M., Fernández-Gutiérrez A., Segura-Carretero A. Phenolic compounds in olive leaves: Analytical determination, biotic and abiotic influence, and health benefits. Food Res. Int. 2015;77:92–108. doi: 10.1016/j.foodres.2015.09.011. [DOI] [Google Scholar]
- 34.Filocamo A., Bisignano C., Mandalari G., Navarra M. In Vitro Antimicrobial Activity and Effect on Biofilm Production of a White Grape Juice (Vitis vinifera) Extract. Evid Based Complement. Altern. Med. 2015;2015:856243. doi: 10.1155/2015/856243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Muscarà C., Smeriglio A., Trombetta D., Mandalari G., La Camera E., Occhiuto C., Grassi G., Circosta C. Antioxidant and antimicrobial activity of two standardized extracts from a new Chinese accession of non-psychotropic Cannabis sativa L. Phytother. Res. 2021;35:1099–1112. doi: 10.1002/ptr.6891. [DOI] [PubMed] [Google Scholar]
- 36.Lowy F.D. Staphylococcus aureus infections. N. Engl. J. Med. 1998;339:520–532. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- 37.Karygianni L., Cecere M., Skaltsounis A.L., Argyropoulou A., Hellwig E., Aligiannis N., Wittmer A., Al-Ahmad A. High-level antimicrobial efficacy of representative mediterranean natural plant extracts against oral microorganisms. Bio. Med. Res. Int. 2014;2014:839019. doi: 10.1155/2014/839019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brahmi F., Flamini G., Issaoui M., Dhibi M., Dabbou S., Mastouri M., Hammami M. Chemical composition and biological activities of volatile fractions from three Tunisian cultivars of olive leaves. Med. Chem. Res. 2012;21:2863–2872. doi: 10.1007/s00044-011-9817-8. [DOI] [Google Scholar]
- 39.Khalil M.M., Ismail E.H., El-Baghdady K.Z., Mohamed D. Green synthesis of silver nanoparticles using olive leaf extract and its antibacterial activity. Arab. J. Chem. 2014;7:1131–1139. doi: 10.1016/j.arabjc.2013.04.007. [DOI] [Google Scholar]
- 40.Bao J., Zhang D.W., Zhang J.Z., Huang P.L., Huang P.L., Lee-Huang S. Computational study of bindings of olive leaf extract (OLE) to HIV-1 fusion protein gp41. FEBS Lett. 2007;581:2737–2742. doi: 10.1016/j.febslet.2007.05.029. [DOI] [PubMed] [Google Scholar]
- 41.Lee-Huang S., Zhang L., Huang P.L., Chang Y.T., Huang P.L. Anti-HIV activity of olive leaf extract (OLE) and modulation of host cell gene expression by HIV-1infection and OLE treatment. Biochem. Biophys. Res. Commun. 2003;307:1029–1037. doi: 10.1016/S0006-291X(03)01292-0. [DOI] [PubMed] [Google Scholar]
- 42.Lee-Huang S., Huang P.L., Zhang D., Lee J.W., Bao J., Sun Y., Chang Y.T., Zhang J., Huang P.L. Discovery of small-molecule HIV-1 fusion and integrase inhibitors oleuropein and hydroxytyrosol: Part I. Fusion inhibition. Biochem. Biophys. Res. Commun. 2007;354:872–878. doi: 10.1016/j.bbrc.2007.01.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee-Huang S., Huang P.L., Zhang D., Lee J.W., Bao J., Sun Y., Chang Y.T., Zhang J., Huang P.L. Discovery of small-molecule HIV-1 fusion and integrase inhibitors oleuropein and hydroxytyrosol. Part II. Integrase inhibition. Biochem. Biophys. Res. Commun. 2007;354:879–884. doi: 10.1016/j.bbrc.2007.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma S.C., He Z.D., Deng X.L., But P.P., Ooi V.E., Xu H.X., Lee S.H., Lee S.F. In vitro evaluation of secoiridoid glucosides from the fruits of Ligustrum lucidum as antiviral agents. Chem. Pharm. Bull. 2001;49:1471–1473. doi: 10.1248/cpb.49.1471. [DOI] [PubMed] [Google Scholar]
- 45.Micol V., Caturla N., Pérez-Fons L., Más V., Pérez L., Estepa A. The olive leaf extract exhibits antiviral activity against viral haemorrhagic septicaemia rhabdovirus (VHSV) Antivir. Res. 2005;66:129–136. doi: 10.1016/j.antiviral.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 46.Isaacs C.E., Wen G.Y., Xu W., Jia J.H., Rohan L., Corbo C., Di Maggio V., Jenkins E.C., Jr., Hillier S. Epigallocatechin gallate inactivates clinical isolates of herpes simplex virus. Antimicrob. Agents Chemother. 2008;52:962–970. doi: 10.1128/AAC.00825-07. [DOI] [PMC free article] [PubMed] [Google Scholar]