Abstract
Antimicrobial resistance (AMR) is a high priority across countries as it increases morbidity, mortality and costs. Concerns with AMR have resulted in multiple initiatives internationally, nationally and regionally to enhance appropriate antibiotic utilization across sectors to reduce AMR, with the overuse of antibiotics exacerbated by the COVID-19 pandemic. Effectively tackling AMR is crucial for all countries. Principally a narrative review of ongoing activities across sectors was undertaken to improve antimicrobial use and address issues with vaccines including COVID-19. Point prevalence surveys have been successful in hospitals to identify areas for quality improvement programs, principally centering on antimicrobial stewardship programs. These include reducing prolonged antibiotic use to prevent surgical site infections. Multiple activities centering on education have been successful in reducing inappropriate prescribing and dispensing of antimicrobials in ambulatory care for essentially viral infections such as acute respiratory infections. It is imperative to develop new quality indicators for ambulatory care given current concerns, and instigate programs with clear public health messaging to reduce misinformation, essential for pandemics. Regular access to effective treatments is needed to reduce resistance to treatments for HIV, malaria and tuberculosis. Key stakeholder groups can instigate multiple initiatives to reduce AMR. These need to be followed up.
Keywords: antimicrobials, antimicrobial stewardship programs, antimicrobial resistance, healthcare-associated infections, COVID-19, lower- and middle-income countries, misinformation, patient initiatives, surgical site infections, vaccines
1. Introduction
Antimicrobial resistance (AMR) is a high-priority area across all countries, increasing morbidity, mortality and costs [1,2,3,4,5,6,7], with several studies documenting the clinical and economic impact of AMR [2,3,8,9,10,11,12,13,14,15]. There is considerable resistance to commonly prescribed antibiotics among low- and middle-income countries (LMICs) including Bangladesh, with resistance rates continuing to rise across countries [16,17,18]. Rising AMR rates need to be urgently addressed, with the World Bank (2017) believing that even in a low-AMR scenario, the loss on world output due to AMR could exceed US$1 trillion annually after 2030, and potentially up to US$3.4 trillion annually, unless addressed, equivalent to 3.8% of annual Gross Domestic Product (GDP) [19]—with the costs of AMR appreciably exceeding those of the antibiotics prescribed [13].
Rising AMR rates are due to a number of factors including rising antimicrobial utilization especially in LMICs [20,21], with the overuse of antimicrobials linked with increasing AMR [19,22,23]. Alongside this, the availability of substandard, spurious or falsified antibiotics, high rates of self-medication especially in LMICs often for self-limiting diseases, the extensive use of antimicrobials to prevent and treat diseases in animals considering that their manure is often used in food production and for aquaculture, water pollution, lack of hand hygiene and proper sanitation as well as travelling between countries enhances AMR rates [11,19,21,23,24,25,26,27,28,29,30,31,32,33,34]. In their recent paper, Van Boeckel et al. (2019) found nearly a 3-fold increase in bacteria showing resistance above 50% to antimicrobials in chickens and pigs in LMICs between 2000 and 2018 [35], with resistance rates expected to rise unless addressed.
Low vaccination rates against disease have also enhanced antimicrobial use and AMR, with current concerns that vaccination rates are being further compromised by the COVID-19 pandemic, with its resultant impact on patient movement and available facilities as a result of lockdown and on other measures [11,21,36,37,38,39,40,41,42], exacerbated by vaccine hesitancy generally alongside misinformation [43,44]. We are though seeing approaches to combat this including mobile vaccination clinics [45].
The general overuse of antimicrobials has been worsened by the COVID-19 pandemic [3,46,47,48], with concerns with differential diagnosis between infections such as tuberculosis (TB) and bacterial upper respiratory tract infections (URTIs) from influenza, coughs, fever and COVID-19 [49,50]. Published studies suggest that over 70% of patients with COVID-19 received antibiotics, including broad-spectrum antibiotics, even when not clinically indicated [41,51,52]. Typically, less than 10% of COVID-19 patients appear to have bacterial or fungal infections [53], with some studies suggesting that only 3.2% of COVID-19 infections are co-infections warranting antibiotics [21,54]. In their recent meta-analysis, Langford et al. (2021) found that overall three-quarters of patients with COVID-19 were prescribed antibiotics, which is significantly higher than the estimated prevalence of bacterial co-infections [21,55]. Consequently, the authors concluded that unnecessary antibiotic use is high in patients with COVID-19 [55], which will enhance AMR rates.
Concerns with rising AMR rates and the consequences have resulted in international, regional, national, and local strategies to try and reduce the rises [12,56,57,58,59,60,61,62,63,64,65,66,67,68]. National initiatives include National Action Plans (NAPs) under the direction of the WHO, building on its Global Action Plan [59,60,69,70,71,72,73,74]. We are already seeing countries assess their progress of NAPs against agreed targets, and this will continue [63,75,76].
Alongside this, there are concerns with multi-resistant microorganisms and the implications for patients with TB, human immunodeficiency virus (HIV), malaria, and typhoid, as well as those with co-infections [77]. Early treatment for patients with co-infections with HIV and drug-resistant TB is essential to reduce the significant risk of death [77,78]. There is also ongoing research to better predict patients with MDR-TB and improve future care [79]. Treatment centers, especially in rural areas, are important to monitor treatment progress and provide access to medicines, to enhance successful management and reduce future cases [80].
Overall, we are aware that coordinated activities among all key stakeholders can address over-utilization of antibiotics. Multiple demand-side measures including education of all key stakeholders, prescribing restrictions, and follow-up in pharmacies stabilized or reduced the utilization of antibiotics in former Soviet Union republics including Azerbaijan, the Republic of Srpska, Bosnia and Herzegovina, and Slovenia versus Poland that failed to instigate such measures [61,62,81,82]. In Poland, there was a small but statistically significant average annual increase in total antibiotic consumption between 2007 and 2016 [82].
We are also aware that there are concerns with high prevalence rates of hospital-associated infections (HAIs) in a number of LMICs, with major challenges surrounding the appropriate use of antibiotics to reduce surgical site infections (SSIs) [83,84,85]. The instigation of ASPs in hospitals, including undertaking point prevalence surveys (PPS) to identify areas for quality improvement programs, can improve future antibiotic use and reduce AMR [3,86,87,88,89,90,91,92,93,94,95,96]. There are though manpower and resource challenges with implementing ASPs in hospitals in LMICs [97]. Having said this, successful ASPs have been introduced in hospitals in LMICs to improve antimicrobial prescribing providing guidance to others [3,76,87,98]. Successful programs have also been introduced in ambulatory care in LMICs to improve prescribing and dispensing of antibiotics for essentially viral infections such as acute respiratory infections (ARIs) and including URTIs [64,99,100]. We are also seeing new technologies being developed, including Apps, to provide prescribing advice and other information to physicians and patients to reduce inappropriate use of antibiotics [101]. However, there are concerns with variable adherence to guidelines, possibly exacerbated by guidelines not always considering local resistance patterns, as well as other activities such as community engagement to improve patient care in practice [64,102,103].
We would like to build on these issues and challenges to discuss potential ways to decrease future AMR rates among LMICs. We have chosen LMICs as they currently have the greatest increase in antimicrobial use, the highest rate of self-purchasing of antibiotics, as well as the highest rate of antimicrobial use in hospitals [20,64,104,105]. In addition, mortality rates from AMR are likely to be highest among LMICs, including those in Africa and Asia, by 2050 unless addressed [11,25]. Effectively tackling AMR is crucial for the future of all countries, exacerbated by few new antibiotics currently being developed; however, this is counterbalanced to some extent by developments in vaccine technology [10,19,32,106]. We hope our review paper will consolidate current knowledge in key areas to provide guidance on potential ways forward among all key stakeholders across LMICs to reduce AMR rates, especially where there are continued concerns.
2. Materials and Methods
We undertook a narrative review of published papers to document potential ways to enhance appropriate prescribing and dispensing of antimicrobials. We purposely did not undertake a systematic review as an appreciable number of these have already been conducted on different aspects to improve future prescribing and dispensing of antimicrobials [85,86,92,100,104,105,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134]. In addition, we did not document the number of publications by country and sector as we are aware that some countries are more prolific in their publications than others, and such analyses may bias the actual situation. We did though divide initiatives up into those directed at hospitals compared with those in ambulatory care. We are aware that issues such as self-purchasing of antibiotics are especially important in LMICs as up to 100% of pharmacies in these countries dispense antibiotics without a prescription despite legislation which can account for up to 93% to 100% of all dispensed antibiotics [123,124,129,135,136]. This because our principal aim was to provide possible future guidance to all key stakeholder groups within LMICs for potential debate based on the considerable experiences of the co-authors from multiple LMICs. We have successfully used this approach before to stimulate debate in other priority healthcare areas to provide future guidance [64,85,137,138,139,140,141,142,143,144,145,146,147].
The World Bank classification was used to break countries down into LMICs or upper income countries where applicable [148]. Similar to previous studies conducted by the co-authors, upper-income countries will only be commented upon where pertinent [64,84,146]. This is because we are aware that there are different practices and beliefs, as well as a greater availability of healthcare professionals and resources, in higher- versus middle- and low-income countries. In addition, in LMICs, the costs of medicines can account for up to 60% or more of total healthcare expenditure, much of which will be out of pocket, with potentially catastrophic consequences for families when members become ill [149,150,151,152,153,154].
There can also be long waiting times for patients to see physicians in primary healthcare centers (PHCs) in LMICs, long distances to travel to PHCs, and pharmacists are typically open later in the day, resulting in patients often seeking their advice as alternatives to physicians [155,156,157,158,159,160]. This is helped by the fact that community pharmacists are typically trusted by patients and less expensive than physicians, with patients not having to pay physician fees when the need arises [99,135,157,161]. However, we are aware that there can be concerns with the knowledge of pharmacists and their assistants regarding antibiotics and AMR [159,162,163,164].
When documenting interventions that have been undertaken to enhance appropriate prescribing and dispensing of antimicrobials, we will break these down into the 4Es to enhance understanding and comparisons, namely Education, Engineering, Economics and Enforcement [165,166]. Education includes developing guidelines or formularies, with adherence to well-constructed guidelines increasingly seen as demonstrating good quality care [147,167,168,169,170,171,172]. This also includes educational and monitoring activities surrounding the WHO AWaRe list of antibiotics [173,174,175]. Instigating Drug and Therapeutic Committees (DTCs) and ASPs in hospitals to provide educational activities to enhance the rational use of medicines including antimicrobials [97,176,177,178,179] is also classified as education. Engineering includes organizational or managerial interventions which incorporate prescribing and quality targets. These include the percentage of antibiotics prescribed according to agreed guidance and the WHO AWaRe list, the percentage of patients not prescribed prolonged antibiotics to prevent SSIs as well as the percentage of patients reaching agreed target blood pressure levels [147,166,169,175,180,181]. Economics includes financial incentives to physicians, pharmacists, patients or hospitals, for instance providing financial incentives to hospitals to improve their patient safety records and/or reduce payments where there are concerns, incentivizing physicians for attaining agreed prescribing targets including adherence to guidelines, or fining pharmacists for illegally dispensing an antibiotic without a prescription [9,147,166,182,183]. Enforcement includes regulations by law such as banning the dispensing of antibiotics in pharmacies without a prescription, compulsory generic substitution as well as national policies outlining the need for DTCs in hospitals and how their activities should be monitored [100,166,177].
No ethical approval was required for this review as we were not dealing with patients. This is in line with previous similar papers published by the co-authors [64,84,105,138,146,147,184,185].
3. Results
There are a number of key issues that need to be addressed to enhance appropriate antimicrobial use in patients and reduce AMR. We will break our findings down into key areas within hospitals and subsequently ambulatory care before concluding with guidance on potential activities that can be undertaken among all key stakeholder groups to reduce AMR.
Activities that can be undertaken in hospitals include PPS studies to gain baseline data of current utilization patterns to guide future quality improvement programs as part of ASPs and other activities. Initiatives in ambulatory care center around activities to improve prescribing and dispensing of antimicrobials especially for self-limiting infections such as viral URTIs. These are especially important since, as mentioned, we are aware that up to 100% of pharmacies in LMICs dispense antibiotics without a prescription despite legislation, and this can account for up to 93% to 100% of all dispensed antibiotics [123,124,129,135,136]. Having said this, HAIs are considered as the most adverse event in healthcare delivery [104,186].
3.1. Antimicrobial Use in Hospitals
The first consideration within hospitals is to ensure that activities to improve antimicrobial prescribing are given a high priority if this is not already the case. This includes initiatives such as instigating active infection, prevention and control committees (IPC committees) [187,188,189,190,191,192], which can be part of antimicrobial stewardship groups [76,86]. The latter can be part of IPC committees or attached to Drug and Therapeutic Committees (DTCs). In addition, PPS studies can be undertaken and ASPs indicated to improve future antimicrobial use in hospitals (Table 1).
Table 1.
Hospital activities to improve antimicrobial use.
| Activity | Role with Improving Antimicrobial Prescribing |
|---|---|
| IPC committees |
|
| PPS studies |
|
| ASPs |
|
However, there can be issues with the instigation of IPC committees and their effectiveness in hospitals (Table 1), concerns about the knowledge of ASPs among clinicians in LMICs, concerns with resource issues with establishing active ASPs including manpower, as well as a lack of institutional policies in LMICs [97,188,214,215,216]. These issues can be exacerbated by a lack of clear commitment nationally despite the instigation of NAPs, which needs to be addressed to improve future antimicrobial use [213,216].
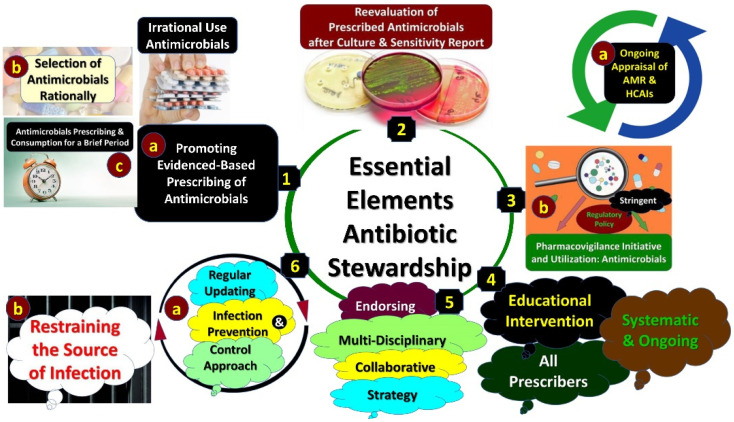
Figure 1 incorporates the core elements of any ASP, with Kakkar et al. (2020) discussing the potential composition of personnel within antimicrobial stewardship groups in LMICs including physicians, pharmacists, microbiologists, infection control personnel as well as hospital administrators [213]. Ideally, there should be a single focal point, with leaders of ASPs typically being either physicians or pharmacists, with pharmacists increasingly helping to drive forward ASPs in LMICs [87,98,171,211,217]. ASPs can also be part of DTC activities if needed in some hospitals [211].
Figure 1.
Core elements of any antimicrobial stewardship program.
Box 1 contains a number of indicators that have been used to assess the quality of antimicrobial prescribing within hospitals.
Box 1. Possible indicators that have been used in LMICs to assess the quality of antimicrobial prescribing (adapted from [88,170,171,193,211,218,219,220]).
Availability, ready access and subsequent compliance to local or regional treatment guidelines for key infections. This includes the availability of antibiograms within hospitals to guide empiric use whilst waiting for culture and sensitivity test (CST) findings as well as a potential list of restricted antibiotics depending on physician experience
Whether antibiotics prescribed are included in the hospital formulary/national essential medicine lists
Rationale (indication) for antimicrobial use documented in the patient’s notes
Whether therapy was empiric or targeted based on CST findings
Documentation of the dose in the patient’s notes
Documentation of a stop/review date for given antibiotics in the patient’s notes. This can include the rate of de-escalation to a narrower-spectrum antibiotic where appropriate
Documentation of any missed doses of antibiotics
Extent of IV to oral switching (based on the stability of the gastrointestinal tract)
Duration of surgical prophylaxis
Changes in antibiotic use over time in terms of Defined Daily Doses/100(0) bed days and/or days of therapy/patient-days
Whether active antimicrobial stewardship groups and/or drug and therapeutic committees/Infection Prevention and Control groups in the hospital
Multiple activities to reduce HAIs include encouraging greater hand hygiene, regular reviews surrounding the use and care of catheters, instigating appropriate strategies to reduce ventilator-associated pneumonia including subgluteal space suctioning before each position change, chlorhexidine gluconate bathing as well as active monitoring of agreed guidelines to reduce HAIs [132,221,222]. A number of initiatives have been undertaken to improve hand hygiene in LMICs including those orchestrated by the WHO and others providing direction [223,224].
Multiple activities can also be undertaken to improve antibiotic utilization to reduce SSIs among LMICs [84]. Recent publications have documented that the number of patients receiving their first dose of antibiotics within 60 min of the first incision, with a second dose administered for longer procedures, can be as low as 6.7% of surgical patients in LMICs [84,108,225,226]. This is a concern as studies have shown the risks of SSIs are almost five times higher when antibiotics are administered more than two hours prior to the first incision and almost doubled when administered after the first incision [84,227]. There are also concerns that extending prophylaxis beyond one day, which is frequent among LMICs with for instance a mean up to 8.7 days post-operatively in Nigeria, does not appear to improve outcomes whilst concurrently increasing the potential for acute kidney injury, Clostridium difficile infections, and AMR as well as increasing costs [84,228,229,230,231,232,233]. Vicentini et al. (2019) in their published study found that adequate antibiotic choices and length of administration were associated with significantly reduced risks of SSIs [234], with a number of approaches successfully undertaken in LMICs to improve antibiotic use surrounding the prevention of SSIs [84].
3.1.1. Antimicrobial Stewardship Programs (ASPs) and Other Activities to Improve Antimicrobial Utilization in Hospitals
ASPs as well as other interventions can improve overall utilization of antimicrobials in hospitals as well as reduce costs alongwith improved hand hygiene and other activities to reduce HAIs and SSIs (Table 1) [223,235,236,237]. There are a number of areas that can be initial targets for ASP programs to gain their acceptance among key stakeholder groups in hospitals to address barriers [211,212,213]. These include developing guidelines for specific indications and monitoring subsequent usage against these guidelines, as well as developing prompts for physicians to regularly review antibiotic prescriptions.
Table 2 summarizes some of the activities that have been undertaken in LMICs to improve antimicrobial utilization in hospitals, which build on reviews in predominantly high-income countries including the use of computerized decision support systems [236,238]. This includes (Table 2) activities surrounding SSIs and their impact to provide future guidance. Activities principally centre on educational initiatives [93], with educational initiatives known to enhance understanding of ASPs in LMICs given concerns about knowledge and activities among students and physicians in a number of LMICs [214,215,239,240].
Table 2.
Summary of published studies across LMICs documenting the impact of ASP interventions including those surrounding SSIs.
| Author, Country and Year | Brief Details of the Intervention | Impact of the Intervention |
|---|---|---|
| Bozkurt et al., Turkey, 2014 [241] | Principally educational interventions to improve appropriate antibiotic use for SSIs. These included:
|
|
| Hou et al., China, 2014 [242] | A number of interventions were undertaken to improve antibiotic utilization in ICUs as part of ASPs. These included:
|
|
| Amdany et al., Kenya, 2014 [243] | Principally an educational initiative to enhance the use of oral vs. IV metronidazole including education, audit and feedback |
|
| Yang et al., China, 2014 [244] | Education and Engineering interventions to improve antibiotic use for SSIs. These included the Introduction of a Drug Rational Usage Guideline System (DRUGS) vs. paper-based guidelines to enhance adherence to surgical prophylaxis guidelines |
|
| Okumura et al., Brazil, 2015/2016 [245,246] | Primarily Education comparing the outcomes from two different ASP approaches among patients in the general ward and ICU:
|
|
| Saied et al., Egypt, 2015 [247] | Principally Education and Engineering to improve antibiotic use of SSIs. These included
|
|
| Ntumba et al., Kenya, 2015 [248] | Principally Education and Engineering to improve the use of antibiotics in relation to SSIs. These included:
|
|
| Apisarnthanarak et al., Thailand, 2015 [249] | This ASP principally involved Education with a 12 h training course run by infectious diseases clinical pharmacists (IDCPs) with physicians looking after patients in medical wards coupled with an option for infectious diseases consultations (IDCs), daily rounds with ICDPs, or both if wished, with a control (Usual Standard of Care) group | For patients with input from the IDCP group or the IDCP plus IDC group vs. controls, they were:
|
| Boyles et al., South Africa, 2017 [250] |
|
|
| Brink et al., South Africa, 2017) [251] | Education and Engineering. Key activities driven by hospital pharmacists included:
|
|
| Allegranzi et al., Kenya, Uganda, Zambia, and Zimbabwe, 2018 [252] | Principally Education and Engineering to improve antibiotic prescribing for SSIs. This included:
|
|
| Khdour et al., Palestine, 2018 [253] |
|
|
| Abubakar et al., Nigeria, 2019 [254] | Principally Education and Engineering to improve antibiotic prescribing for the prevention of SAP. This included:
|
|
| Karaali et al., Turkey, 2019 [255] | Multiple activities to improve antibiotic prescribing for SSIs including Education, and Engineering. These incorporated:
|
|
| Mahmoudi et al., Iran, 2019 [256] | Principally Education and Engineering to improve SAP:
|
|
| Xiao et al., China, 2020 [257] | Multiple activities include Education, Engineering, Economics and Enforcement over a 6-year campaign:
|
Over the six years—2016 vs. 2010:
|
| Mardani et al., Iran, 2020 [258] | Principally education for this ASP comprising:
|
|
| van den Bergh et al., South Africa, 2020 [259] | Principally education to improve compliance to agreed guidelines:
|
|
We are aware that ASPs have been successfully instigated in Accident and Emergency Departments in hospitals. However, this typically applies to higher-income countries [134,260,261].
3.1.2. Dealing with Antimicrobial Shortages in Hospitals
Other strategies that antimicrobial stewardship groups and DTCs can undertake in hospitals is to develop effective approaches to address antimicrobial shortages, with medicine shortages being an increasing concern across countries [145,262,263,264]. Shortages of antimicrobials can lead to administering less effective, more toxic, and costlier antimicrobials as well as potentially increasing AMR rates [264,265,266]. One way to address this is for key personnel in hospitals, including those who are part of ASTs, to agree in advance therapeutic interchange strategies [264]. This can include consultations between prescribers and hospital pharmacists before alternative antibiotics are administered [264].
Emergency procurement procedures should also be in place in hospitals alongside regular reviews of stock levels in wards, exacerbated by the current COVID-19 pandemic [263,267]. Available resources and funding is also essential to reduce the possibility of antimicrobial shortages in hospitals. The Bamako Initiative in sub-Saharan Africa is one such initiative adopted by Ministers of Health to increase access to medicines including antimicrobials through revolving drug funds [268,269]. However, sustaining earlier gains with this initiative remains a challenge, and alternative drug financing schemes are essential to ensure physicians and patients can access appropriate antimicrobials in hospital to improve outcomes and reduce length of stay [270].
3.2. Antimicrobial Use in Ambulatory Care
We will principally concentrate on ARIs as these are the most common infections seen in ambulatory care in LMICs and where the highest rate of inappropriate prescribing and dispensing takes place [64,102,271,272,273]. Suboptimal management of ARIs in LMICs is exacerbated by a number of issues including social-cultural issues, diagnostic uncertainty, clinical competency and misconceptions as well as from commercial, patient and time pressures [64,114,129,133,274,275,276].
Consequently, there are a number of strategies that can be undertaken in ambulatory care across LMICs to address current issues to improve future antimicrobial utilization. These can principally be divided into those impacting on the prescribing of antimicrobials by physicians for what are essentially viral infections, those strategies that can reduce inappropriate dispensing of antibiotics without a prescription as well as those that can address concerns with multi-resistant organisms for infections such as malaria and TB. In addition, there are a number of strategies that can be implemented to improve vaccination rates including during pandemics.
Improving antimicrobial utilization in ambulatory care for ARIs is especially important in the current pandemic, with, as mentioned, published studies suggesting that over 70% of patients with COVID-19 are receiving antibiotics, including broad-spectrum antibiotics, even when not clinically indicated [51,52], with perhaps as low as 3.2% of infections in ambulatory care warranting antibiotics [21,54].
3.2.1. Initiatives among Physicians to Improve Antibiotic Prescribing
A number of initiatives have been undertaken across LMICs to improve physician prescribing of antibiotics for ARIs. Table 3 summarizes examples of the many multiple activities that have been undertaken in LMICs to improve antimicrobial prescribing especially for ARIs, principally in education, and their impact.
Table 3.
Initiatives to improve antibiotic utilization among physicians in ambulatory care in LMICs especially for ARIs.
| Author, Country, Year | Intervention and Impact | Impact |
|---|---|---|
| Teng et al., Malaysia, 2006 [277] | Education—Academic detailing from the resident family medicine specialist accompanied by an information leaflet |
|
| Shrestha et al., Nepal, 2006 [278] | Principally Education—7 health posts and 33 subhealth posts were stratified by type with health workers. The intervention was based on 5 days of training on the adapted Practical Approach to Lung Health (PAL) guidelines and their use |
|
| Awad et al., Sudan, 2006 [279] |
|
|
| Kafle et al., Nepal, 2009 [280] |
|
|
| Yip et al., China, 2014 [281] | Principally Economics: In Ningxia Province, a randomized study was undertaken to evaluate the effects of capitation with pay-for-performance on antibiotic prescribing practices, health spending, outpatient visit volumes, and patient satisfaction |
|
| Boonyasiri et al., Thailand, 2014 [282] |
Principally Education including:
|
The multifaceted program resulted in:
|
| Wei et al., China, 2019 [273] |
|
This multifaceted approach appreciably reduced prescribing rates for antibiotics (ABR) in children with URTIs:
|
| Tay et al., Malaysia, 2019 [283] | Principally Education—Educational toolkits included a training module for HCPs on URI and acute diarrhoea involving:
|
Appreciable reduction in antibiotic prescribing:
|
Among LMICs, there is an absolute imperative to develop actionable quality and patient safety indicators that better represent current prescribing compared with the traditional WHO/INRUD criteria [284]. This reflects the rise in the number of patients in LMICs, especially sub-Saharan Africa, with both infectious and non-infectious communicable diseases (NCDs) as well as combinations of infectious diseases such as HIV and TB and combinations of infectious diseases and NCDs [285,286,287,288,289]. Consequently, there is a need to develop more specific quality and patient safety indicators for LMICs to improve future antimicrobial prescribing. This can be achieved using agreed and common coding of data to enable coordinated and transferable learning between countries including patients attending ambulatory care clinics in sub-Saharan Africa. This will be the subject of future research activities among the group.
These activities build on recent studies, including those undertaken in sub-Saharan Africa, suggesting that adherence to guidelines is a better indicator of the quality of prescribing in ambulatory care in LMICs compared with the current WHO/INRUD criteria [167,169,181,290]. Having said that, it is important that there is only one national reference guideline since contradictions can arise when there are multiple guidelines within a country [291]. It is also essential that any guidelines produced must be readily available in multiple media formats including online and downloadable, as well as in print. National guidelines also need to be combined with educational inputs and auditing of prescribing practices to further improve future prescribing [114,172,181,290,292,293].
Regular updates of any guidelines, especially online, are also essential to improve adherence rates, and any updates need to be rapidly disseminated. This is being achieved among a number of sub-Saharan African countries through mobile technologies [101,293], providing guidance to other LMICs.
We are aware though that over prescribing of antibiotics can be appreciably more common in telemedicine consultations than in face-to-face visits [294,295]. Consequently, these consultations also need to be part of any future quality initiatives.
3.2.2. Initiatives to Reduce Inappropriate Dispensing of Antimicrobials among Pharmacists in the Community
There are concerns with the extent of inappropriate dispensing of antimicrobials in community pharmacies and drugs stories across LMICs, especially with, as mentioned, such utilization accounting for up to 93% to 100% of all dispensed antibiotics [123,124,129,135,136].
These concerns can be exacerbated by variable knowledge regarding antibiotics and AMR among community pharmacists [162,296,297,298]. Having said this, there are key issues in rural settings in LMICs that need to be considered by Governments and others when reviewing strategies to reduce inappropriate dispensing of antimicrobials. These include the fact that pharmacists maybe the only healthcare professional available and they are trusted [49,135,156,299]. There can also be major issues with affordability among patients in LMICs. Meeting both physician fees and the cost of medicines can have catastrophic consequences for individuals and families in LMICs [151,152,300]. Trained pharmacists, assisted by guidelines, can reduce inappropriate self-purchasing of antibiotics in LMICs as well as be leaders of ambulatory care ASPs in their countries to improve future antibiotic utilization especially for infections that are essentially viral [99,157,301].
Strategies to successfully reduce inappropriate self-purchasing of antimicrobials range from having well-educated pharmacists offering advice (Education) to patients as seen in Kenya, regulations by law restricting the purchasing of antimicrobials without a prescription coupled with fines (Economics and Enforcement), or a combination of these as seen in the Republic of Srpska (Education, Economics and Enforcement) [99,100,157,183].
The extent of ‘enforcement’ including the potential for fines or closure of pharmacies is vital as this impacts on the outcome of this intervention (Table 4). For instance, in Venezuela, the Government implemented policies to limit self-purchasing of antibiotics of three antibiotic groups. However, there were no public awareness campaigns and ‘Enforcement’ was only via government publications with no follow up. As a result, there was no decrease in antibiotic utilization levels but, in fact, the opposite with an increase [302]. Similarly, in Colombia, whilst initial enforcement of the law in 2005 had a modest impact on overall sales in the first three years (−1.00 DDDs/1000 inhabitants per day), a follow-up study five years after implementation found that 80.3% of pharmacies were still not complying with the law due to lax monitoring, prompting calls for greater enforcement [302,303]. This contrasts with an appreciable reduction in antibiotics dispensed without a prescription in a number of countries following multiple interventions, e.g., Saudi Arabia (Table 4).
Table 4.
Examples of initiatives to reduce self-purchasing of antibiotics in pharmacies predominantly in LMICs (adapted from [64,100,183]).
| Country and Year | Activity |
|---|---|
| Brazil and Mexico, 2013—private pharmacies [307] | Enforcement—assessing the impact of legislation to ban self-purchasing of antibiotics on dispensing patterns between 2007 and 2012 in private pharmacies in Brazil and Mexico (has always been the case among public pharmacies in Brazil [137,308]) Variable results seen
|
| Brazil—Both private and public pharmacies—2015 to 2017 | Enforcement:
|
| Thailand, 2015 [311] |
|
| Republic of Srpska, 2017 [157,312] |
|
| Kenya—2018 [99] and 2021 [313] |
|
| Saudi Arabia, 2020 [183] |
|
| India, Malaysia and Vietnam, 2021 [153,299] |
|
We see similar examples in other disease areas where the intensity of follow-up of prescribing restrictions (enforcement) appreciably impacts on their effectiveness and outcome in practice [304,305,306].
However, it can be difficult in practice in a number of LMICs to enforce regulations banning self-purchasing of antibiotics due to manpower issues as well as shortages of antimicrobials within public healthcare facilities. Such activities may also be counter-productive in rural areas in LMICs and we have seen that trained pharmacists can help reduce inappropriate dispensing of antibiotics.
Monitoring pharmacy dispensing activities through mobile technologies as well as potentially implementing information technology (IT) surveillance systems to track antibiotics through the supply chain are also potential strategies to reduce inappropriate dispensing [99,135].
Adoption of such tactics may mean re-looking at pharmacists’ knowledge regarding AMR and its causes, with the potential for increased training if needed starting in pharmacy school and continuing post-qualification [159,162,314,315]. In addition, potentially ensuring through regulation and other mechanisms that any antibiotic dispensed for ARIs and other common conditions are principally from the Access group of antibiotics and not from the WHO ‘Watch’ or ‘Reserve’ list [173,174].
3.2.3. Initiatives to Reduce Inappropriate Dispensing of Antimicrobials among Patients and the Public
The impact of programs to reduce inappropriate prescribing and dispensing of antimicrobials for essentially viral infections such as ARIs can be enhanced by concurrently targeting patients and the public. This is because multiple studies have shown that some people can put considerable pressure on healthcare professionals to prescribe and dispense antibiotics for ARIs [314,316,317,318]. Knowledge about antibiotics and AMR can vary though among patients and the public, and they can have strong beliefs about the effectiveness of antibiotics for them even for viral infections, which adds to the pressure to prescribe or dispense antimicrobials for ARIs [318,319,320,321,322,323,324].
Similar to the situation with physicians and pharmacists, multifaceted approaches are needed to change attitudes and behaviors among patients and caregivers [325,326]. However, such programs have had variable degrees of success for countries including both high-income countries and LMICs [318,327,328,329]. Published studies, particularly among high-income countries, suggest that a number factors can enhance the success of any antibiotic awareness campaign. These include (i) the use of carefully designed simple key messages; (ii) targeting a wide audience including patients and their families; (iii) designing key messages for all key stakeholder groups for completeness; (iv) using mass media including social media to raise awareness; and (v) repeating key messages especially for new parents and others [318,328,330,331,332].
Two systematic reviews have suggested that campaigns among patients can significantly reduce antibiotic utilization. McDonagh et al. (2018) found that parent education reduced antibiotic prescribing for ARIs by 21% [116]. Cross et al. (2017), mainly including studies from high-income countries, also found that the majority of studies reported reductions in antibiotic utilization. The reduction was greater than 14% in the prescribing of antibiotics for RTIs, up to a maximum reduction of 30% [318].
However, the impact of patient education initiatives in LMICs have not yet been fully elucidated, nor their costs and cost-effectiveness [115]. Consequently, caution is still needed before fully embarking on such campaigns in LMICs unless these are part of research programs.
3.2.4. Initiatives to Address MDR Organisms for HIV, Malaria and TB
There are concerns that patients with TB living in rural areas, and without regular supply of medicines, will have poorer treatment outcomes [80,333]. Potential reasons for this include insufficient funds for transport to treatment centers despite the medicines often being provided free of charge alongwith missing out on regular reviews of medicine use addressing concerns including compliance with prescribed medicines, similar to the situation with NCDs [80,158,334]. Poorer outcomes and lack of adherence to treatments will enhance resistance rates [80,335].
The overall burden of TB has been declining at an annual average of 2%; however, the number of patients with drug-resistant TB (DR-TB) is increasing. Aminoglycosides, such as kanamycin, and some polypeptides, such as capreomycin, are used to treat DR-TB. However, whilst aminoglycosides are inhibitors of prokaryotic protein synthesis at commonly accepted therapeutic concentrations, they can affect the protein synthesis of cells at larger concentrations, leading to toxicity such as ototoxicity, vestibulotoxicity, and nephrotoxicity [336]. Hearing loss though can now be monitored using an ototoxicity grading system within a mobile app to assist health professionals in assessing patients for ototoxicity. Subsequently, monitoring progression of hearing loss to improve planning for any changes in care [337]. Such developments are likely to continue to improve the future use of aminoglycosides.
There are also concerns with rising resistance rates to antimicrobials used to treat malaria since for instance when resistance of P. falciparum to sulfadoxine-pyrimethamine was greater than 10%, the risk ratio for malaria infections was 5.9 times higher compared to a period of time with low resistance rates, and the risk of death from malaria 10.8 times higher [6,338].
Developing resistance to treatments for HIV also increases mortality with studies from sub-Saharan Africa indicating that when pre-treatment drug-resistance levels are evident but below 10%, AMR will be responsible for an estimated 710,000 AIDS deaths and 380,000 new infections by 2030. This increases to an estimated 890,000 AIDS deaths and 450,000 new infections by 2030 attributable to HIV when resistance rates are above 10% [6,339].
Strategies to address concerns with resistance to treatments for HIV, malaria and TB include initiatives to ensure regular access to treatment facilities, including expanding these in rural areas given concerns with travel costs and time off work, as well as regular access to treatments ideally provided free of charge. Alongside this, regular monitoring of adherence rates knowing adherence can be a major issue in some patients [80,340,341,342]. Strategies to improve adherence rates include patient and staff education addressing pertinent issues of anxiety and depression with their condition, encouraging clinic visits, addressing socioeconomic factors where possible as well as increasing the use of digital technologies including mobile phones and video-observed therapy [341,342,343,344,345,346,347,348].
Concurrent with this, we are aware of groups in South Africa undertaking research on HIV reservoirs and using locally discovered potent, broadly neutralizing antibodies to neutralize HIV in the reservoir to render patients non-infectious as a cure strategy [349]. Recent promising data from primates indicate the potential value of this approach and clinical trials are being planned.
We are also aware of ongoing research that provides an expansive dataset of compounds that could be redirected for antimalarial development and also point towards proteins that can be targeted in multiple parasite life cycle stages to improve the management of patients with malaria resistant to current treatments [350]. We will be monitoring these developments and their implications.
3.2.5. Programs to Address Concerns with Vaccine Uptake Including Misinformation as Well as Other Situations Adversely Affecting Antimicrobial Use
Reduced vaccine uptake including vaccine hesitancy fueled by misinformation is a concern in view of the impact on AMR, morbidity and mortality (Table 5) [10,37].
Table 5.
Positive impact of vaccine uptake and concerns with vaccine hesitancy.
| Disease Area | Impact |
|---|---|
| Hib conjugate vaccine and pneumococcal polysaccharide vaccine |
|
| S. pneumoniae and rotavirus |
|
| COVID-19 |
|
This increasing recognition of the role of vaccines in reducing antimicrobial use and AMR is reflected in the recent WHO Action Framework to enhance current vaccination rates, encourage greater knowledge and dissemination on the role of vaccines to reduce AMR as well as encourage the development of new vaccines to further reduce AMR [358,359].
Addressing mis- and dis-information surrounding vaccines is critical to reduce future morbidity and mortality arising from pandemics [360,361]. We are already seeing social media companies such as Facebook and Twitter tightening up on accounts that repeatedly publish misinformation about COVID-19, building on activities among some African countries to fine companies for spreading misinformation [36,362], and such activities are likely to continue. This builds on activities by the WHO to join forces with Governments to address the level of misinformation surrounding COVID-19 as an example [363] as well as Governments funding projects to specifically address misinformation surrounding vaccines for COVID-19 to enhance their uptake [364,365]. Inclusivity is essential to engage with community groups and patient organizations [366], and ensure that clear public health messaging is delivered that is sensitive to age, culture, socioeconomic context and education, and to build population resilience against AMR behaviors [367].
Potential ways forward, building on the activities of the WHO and Governments, especially with proposed medicines for COVID-19 including hydroxychloroquine, lopinavir/ritonavir and remdesivir that failed to live up to their preceived promise [368,369,370,371,372,373], include instigating a culture of evidence-based medicine among all key stakeholder groups starting in universities and continuing post-qualification [36,166,374]. Such activities would have helped reduce shortages and price rises of hydroxychloroquine and antibiotics arising from the initial studies as well as help reduce inappropriate prescribing of antibiotics [41,49,153,166].
Governments and others working with pertinent patient organizations within countries also need to proactively address misinformation [366], especially if, as mentioned, this increases future morbidity, mortality and costs, as seen with misinformation regarding hydroxychloroquine and unwarranted fears about vaccines. Transparent communication and appropriate public health messaging, in collaboration with patient groups and community leaders, are key for increasing the trust of the community and improving vaccination rates where concerns.
3.3. Suggested Activities among All Key Stakeholder Groups to Improve Future Antibiotic Utilization
Suggested strategies to improve the utilization of antimicrobials among LMICs and thereby reduce AMR have been divided into short- and long-term initiatives to provide future guidance (Table 6). These build on the potential for expanding the use of electronic health records and electronic prescribing as well as digital technologies, including clinical decision support systems (CDSS), to improve appropriate use of antibiotics across countries and sectors in LMICs [101,238,375,376]. Such developments should be welcomed given the increasing complexity of medical decision making and, as mentioned, the high percentage of patients especially in sub-Saharan Africa with co-morbidities including HIV, malaria and TB, as well as co-morbidities with both infectious and non-infectious diseases.
Table 6.
Suggested strategies for LMICs to improve appropriate utilization of antimicrobials across sectors.
| Time Scale | Potential Strategies |
|---|---|
| Short term |
Health authority/Government—the following (if not already done so):
|
| Longer-term potential strategies | The findings from the situational analyses and ongoing educational activities can be used together with other research findings within each LMIC to develop pertinent long-term strategies for all key stakeholder groups. These include:
|
Effectively addressing inappropriate antibiotic prescribing is particularly challenging since the majority of antibiotic prescribing in LMICs is undertaken by general clinicians and their assistants who are not experts in infection management and with often influence from pharmaceutical companies in the absence of continual professional development [155,172,377,378,379]. This is compounded across Africa due to high workloads as well as limited consultation times and available resources, driven mainly by a shortage of healthcare professionals and the high prevalence of infectious diseases [380]. Recent evidence is promising though about the feasibility and effectiveness of digital tools to improve future antibiotic prescribing and dispensing [101,204,205,381].
4. Discussion
AMR continues to be a high priority across countries in view of its impact on morbidity, mortality and costs [11,15,19], driven by the overuse of antimicrobials across all sectors including in animals and for the treatment of infectious diseases in patients. The overuse of antimicrobials in humans has been exacerbated by the recent COVID-19 pandemic, where, as mentioned, typically less than 10% of COVID-19 patients have bacterial or fungal infections [53], with as low as 3.2% of COVID-19 infections warranting antibiotics [21,54]. There are similar concerns with current low vaccination rates in a number of countries including current vaccine hesitancy surrounding the COVID-19 vaccine alongwith misinformation surrounding treatments for COVID-19, all of which negatively impact on costs and outcomes [36,43,49,153,161,166,360].
Despite issues of resources including personnel, a number of activities can be undertaken in LMICs to improve future prescribing of antibiotics. These center around the instigation of ASPs in hospitals including PPS studies to identify areas for quality improvement, with a number of quality indicators now developed to monitor the appropriateness of future prescribing (Box 1). Strategies to improve future antibiotic prescribing in hospitals include those to reduce inappropriate timing and duration of antibiotic prescribing to prevent SSIs. Table 1 and Table 2 document a number of examples of initiatives that have been successfully instigated in hospitals in LMICs to improve future antibiotic prescribing, thereby providing exemplars to others.
Successful strategies have also been implemented among physicians in LMIC to reduce inappropriate prescribing of antibiotics for essentially viral infections (Table 3), with patients playing a key role. Patients and pharmacists are also important to reduce inappropriate dispensing of antibiotics without a prescription (Table 4). However, recognizing that in rural LMICs, especially where there are high patient co-payment levels, community pharmacists maybe the only healthcare professional accessible and available. Community pharmacists are often more accessible than ambulatory care physicians in LMICs, and there is no need for physician co-pays, which combined with the cost of treatments can be catastrophic for some families [151,152,300].
Healthcare professionals, including community pharmacists, along with patient organizations have a key role to play to encourage evidence-based approaches to healthcare, thereby reducing the impact of misinformation. Misinformation has been a real concern during the current COVID-19 pandemic resulting in for instance reduced uptake of the vaccines and the implications for this and other vaccines (Table 5), with a number of strategies identified to address this (Section 3.2.5).
A number of strategies and initiatives have also been identified to reduce levels of multi-drug resistance organisms to HIV, malaria, and TB, given the resultant impact on morbidity and mortality. Key areas include the provision of treatments free of charge, ready access to healthcare facilities as well as initiatives to enhance adherence to prescribed treatments.
Potential strategies for all key stakeholders were subsequently consolidated into short-, medium- and long-term activities to provide future direction (Table 6). The key is Government commitment through NAPs and other activities to drive forward future initiatives. We will be monitoring the situation given continued concerns with AMR and its resultant impact on mortality as well as GDP.
We are aware of a number of limitations with this review paper. These include the fact that we did not undertake a systematic review for the reasons discussed. We also did not break the number of examples down by country or care setting as our objective was to provide pertinent examples to guide others. In addition, we are aware that some developing countries are more pro-active with their research than others, potentially biasing the findings. Despite these limitations, we believe that our findings and suggestions are robust, providing future direction.
5. Conclusions
In conclusion, reducing AMR should be a high priority across countries given its clinical and economic impact. Reducing AMR rates requires multiple coordinated activities across sectors driven by Governments and others. This is essential given limited new antimicrobials being developed, although compensated to some extent by developments in vaccine technologies. This will also require strategies to address high rates of vaccine hesitance that exist in a number of countries as seen in the recent COVID-19 pandemic. A coordinated approach including all key stakeholder groups is also essential to minimize misinformation and maximize the impact of future interventions to reduce AMR rates.
Abbreviations
| ABR | prescribing rates for antibiotics |
| AMR | Antimicrobial resistance |
| ARIs | acute respiratory infections |
| ASPs | antimicrobial stewardship programs |
| AST | Antimicrobial stewardship team |
| CDC | Centers for Disease Control and Prevention, US |
| CDDEP | Center for Disease Dynamics, Economics and Policy |
| CPD | Continuous professional development |
| CST | culture and sensitivity testing |
| DTCs | Drug and Therapeutic Committees |
| ECDC | European Centre for Disease Prevention and Control |
| 4Es | Education, Engineering, Economics and Enforcement |
| GDP | Gross Domestic Product |
| GPP | Good Pharmacy Practice |
| HAIs | hospital-associated infections |
| HCPs | healthcare professionals |
| Hib | Haemophilus influenza type b |
| HIV | human immunodeficiency virus |
| ICU | intensive care unit |
| IDCPs | infectious diseases clinical pharmacists |
| IDCs | infectious diseases consultations |
| INRUD | International Network for Rational Use of Drugs |
| IPCs | Infection, Prevention and Control committees |
| IV | intravenous |
| LMICs | low- and middle-income countries |
| MDR | multidrug resistant |
| MDR-TB | multidrug-resistant TB |
| NAP | National Action Plans |
| NCDs | non-infectious communicable diseases |
| OECD | Organization for Economic Co-operation and Development |
| OTC | over the counter |
| PHCs | primary healthcare centres |
| PPSs | point prevalence surveys |
| SA | South Africa |
| SAP | surgical antimicrobial prophylaxis |
| SSIs | surgical site infections |
| TB | tuberculosis |
| UK | United Kingdom |
| URIs | upper respiratory infections |
| URTIs | upper respiratory tract infections |
| US | United States of America |
| UTIs | urinary tract infections |
| WHO | World Health Organization |
| XDR-TB | extensive drug-resistant TB |
Author Contributions
Conceptualization, B.G., M.H., J.S. and R.A.S.; methodology, B.G., M.H. and A.K.; data curation, B.G., A.E., M.H., O.O.M., N.S., S.K., Z.S., J.S., I.H., S.I., J.W., R.C.R.M.d.N., I.P.D.G., L.L.N., A.A.A., J.A., R.I., I.A.S., S.O., A.K., I.C., F.K., D.K., O.O.O., A.O., V.M.-P., J.C.M., T.N.T.P., A.C.K., S.C. and J.W.; writing—original, B.G.; writing—review and editing, all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
No ethical approval was needed for this review paper as this study did not involve humans or animals.
Informed Consent Statement
Not applicable for this review paper.
Data Availability Statement
All sources of information have been referenced.
Conflicts of Interest
The authors declare they have no conflict of interest although some do work for health authorities or are advisers to them.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cassini A., Högberg L.D., Plachouras D., Quattrocchi A., Hoxha A., Simonsen G.S., Colomb-Cotinat M., Kretzschmar M.E., Devleesschauwer B., Cecchini M., et al. Attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European Economic Area in 2015: A population-level modelling analysis. Lancet Infect. Dis. 2019;19:56–66. doi: 10.1016/S1473-3099(18)30605-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hofer U. The cost of antimicrobial resistance. Nat. Rev. Genet. 2019;17:3. doi: 10.1038/s41579-018-0125-x. [DOI] [PubMed] [Google Scholar]
- 3.Majumder M.A., Rahman S., Cohall D., Bharatha A., Singh K., Haque M., Gittens-St Hilaire M. Antimicrobial Stewardship: Fighting Antimicrobial Resistance and Protecting Global Public Health. Infect. Drug Resist. 2020;13:4713–4738. doi: 10.2147/IDR.S290835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Founou R.C., Founou L.L., Essack S.Y. Clinical and economic impact of antibiotic resistance in developing countries: A systematic review and meta-analysis. PLoS ONE. 2017;12:e0189621. doi: 10.1371/journal.pone.0189621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ndir A., Diop A., Ka R., Faye P.M., Dia-Badiane N.M., Ndoye B., Astagneau P. Infections caused by extended-spectrum beta-lactamases producing Enterobacteriaceae: Clinical and economic impact in patients hospitalized in 2 teaching hospitals in Dakar, Senegal. Antimicrob. Resist. Infect. Control. 2016;5:13. doi: 10.1186/s13756-016-0114-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang T., Chen X.S. Outcome Impacts due to Pathogen-Specific Antimicrobial Resistance: A Narrative Review of Published Literature. Int. J. Environ. Res. Public Health. 2020;17:1395. doi: 10.3390/ijerph17041395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graham W.J., Morrison E., Dancer S., Afsana K., Aulakh A., Campbell O.M., Cross S., Ellis R., Enkubahiri S., Fekad B., et al. What are the threats from antimicrobial resistance for maternity units in low- and middle-income countries? Glob. Health Action. 2016;9:33381. doi: 10.3402/gha.v9.33381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.KPMG The Global Economic Impact of Anti-Microbial Resistance. [(accessed on 10 May 2021)]; Available online: https://home.kpmg/content/dam/kpmg/pdf/2014/12/amr-report-final.pdf.
- 9.Yusuf M.A. Antimicrobial Stewardship: Bangladesh Perspective. Bangladesh J. Infect. Dis. 2018;5:1–2. doi: 10.3329/bjid.v5i1.37708. [DOI] [Google Scholar]
- 10.Jansen K.U., Anderson A.S. The role of vaccines in fighting antimicrobial resistance (AMR) Hum. Vaccines Immunother. 2018;14:2142–2149. doi: 10.1080/21645515.2018.1476814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O’Neill Tackling Drug-resistant Infections Globally: Final Report and Recommendations. [(accessed on 8 May 2021)]; Available online: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf.
- 12.OECD Health Policy Studies, Stemming the Superbug Tide. [(accessed on 8 May 2021)]; Available online: https://www.oecd-ilibrary.org/sites/9789264307599-en/index.html?itemId=/content/publication/9789264307599-en&mimeType=text/html.
- 13.Shrestha P., Cooper B.S., Coast J., Oppong R., Thuy N.D.T., Phodha T., Celhay O., Guerin P.J., Wertheim H., Lubell Y. Enumerating the economic cost of antimicrobial resistance per antibiotic consumed to inform the evaluation of interventions affecting their use. Antimicrob. Resist. Infect. Control. 2018;7:98. doi: 10.1186/s13756-018-0384-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haque M., Godman B. Potential Strategies to Improve Antimicrobial Utilisation in Hospitals in Bangladesh Building on Experiences Across Developing Countries. Bangladesh J. Med. Sci. 2021;19:355–357. doi: 10.3329/bjms.v19i3.45849. [DOI] [Google Scholar]
- 15.IACG No Time to Wait: Securing the Future from Drug-Resistant Infections. Report to the Sec-Retary-General of the United Nations. April. [(accessed on 10 May 2021)]; Available online: https://www.who.int/antimicrobial-resistance/interagency-coordination-group/IACG_final_report_EN.pdf.
- 16.Urmi U.L., Nahar S., Rana M., Sultana F., Jahan N., Hossain B., Alam M.S., Mosaddek A.S., McKimm J., Rahman N.A., et al. Genotypic to Phenotypic Resistance Discrepancies Identified Involving β-Lactamase Genes, blaKPC, blaIMP, blaNDM-1, and blaVIM in Uropathogenic Klebsiella pneumoniae. Infect. Drug Resist. 2020;13:2863–2875. doi: 10.2147/IDR.S262493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ara B., Urmi U.L., Haque T.A., Nahar S., Rumnaz A., Ali T., Alam M.S., Mosaddek A.S.M., A Rahman N.A., Haque M., et al. Detection of mobile colistin-resistance gene variants (mcr-1 and mcr-2) in urinary tract pathogens in Bangladesh: The last resort of infectious disease management colistin efficacy is under threat. Expert Rev. Clin. Pharm. 2021;14:513–522. doi: 10.1080/17512433.2021.1901577. [DOI] [PubMed] [Google Scholar]
- 18.Ahmed I., Rabbi B., Sultana S. Antibiotic resistance in Bangladesh: A systematic review. Int. J. Infect. Dis. 2019;80:54–61. doi: 10.1016/j.ijid.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 19.The World Bank Final Report—Drug-Resistant Infections. A Threat to Our Economic Future. [(accessed on 8 May 2021)]; Available online: http://documents1.worldbank.org/curated/en/323311493396993758/pdf/final-report.pdf.
- 20.Klein E.Y., Van Boeckel T.P., Martinez E.M., Pant S., Gandra S., Levin S.A., Goossens H., Laxminarayan R. Global increase and geographic convergence in antibiotic consumption between 2000 and 2015. Proc. Natl. Acad. Sci. 2018;115:E3463–E3470. doi: 10.1073/pnas.1717295115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sriram A., Kalanxhi E., Kapoor G., Craig J., Balasubramanian R., Brar S., Criscuolo N., Hamilton A., Klein E., Tseng K., et al. Center for Disease Dynamics, Economics & Policy; Washington, DC: [(accessed on 10 May 2021)]. State of the World’s Antibiotics 2021: A Global Analysis of Antimicrobial Resistance and Its Drivers. Available online: https://cddep.org/wp-content/uploads/2021/02/The-State-of-the-Worlds-Antibiotics-in-2021.pdf. [Google Scholar]
- 22.Llor C., Bjerrum L. Antimicrobial resistance: Risk associated with antibiotic overuse and initiatives to reduce the problem. Adv. Drug Saf. 2014;5:229–241. doi: 10.1177/2042098614554919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morel C.M., Alm R.A., Årdal C., Bandera A., Bruno G.M., Carrara E., Colombo G.L., de Kraker M.E., Essack S., Frost I., et al. A one health framework to estimate the cost of anti-microbial resistance. Antimicrob. Resist. Infect. Control. 2020;9:187. doi: 10.1186/s13756-020-00822-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ayukekbong J.A., Ntemgwa M., Atabe A.N. The threat of antimicrobial resistance in developing countries: Causes and control strategies. Antimicrob. Resist. Infect. Control. 2017;6:47. doi: 10.1186/s13756-017-0208-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dadgostar P. Antimicrobial Resistance: Implications and Costs. Infect. Drug Resist. 2019;12:3903–3910. doi: 10.2147/IDR.S234610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan J.Z., Hassan Z., Tajik M.I. Antibiotic Resistance: Recommendations for Procurement Agencies of Public Sector Hospitals in Pakistan. J. Coll. Physicians Surg. Pak. 2020;30:340–341. doi: 10.29271/jcpsp.2020.03.340. [DOI] [PubMed] [Google Scholar]
- 27.Mohsin M., Van Boeckel T.P., Saleemi M.K., Umair M., Naseem M.N., He C., Khan A., Laxminarayan R. Excessive use of medically important antimicrobials in food animals in Pakistan: A five-year surveillance survey. Glob. Health Action. 2019;12(Suppl. 1):1697541. doi: 10.1080/16549716.2019.1697541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ali M., Irtiqa A., Mahrukh F., Tooba A. Factors leading to acquired bacterial resistance due to antibiotics in Pakistan. Curr. Trends Biotechnol. Microbiol. 2018;1:1–7. doi: 10.32474/CTBM.2018.01.000101. [DOI] [Google Scholar]
- 29.Bokhary H., Pangesti K., Rashid H., El Ghany M.A., Hill-Cawthorne G. Travel-Related Antimicrobial Resistance: A Systematic Review. Trop. Med. Infect. Dis. 2021;6:11. doi: 10.3390/tropicalmed6010011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoque R., Ahmed S.M., Naher N., Islam M.A., Rousham E.K., Islam B.Z., Hassan S. Tackling antimicrobial resistance in Bangladesh: A scoping review of policy and practice in human, animal and environment sectors. PLoS ONE. 2020;15:e0227947. doi: 10.1371/journal.pone.0227947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Islam M.A., Islam M., Hasan R., Hossain M.I., Nabi A., Rahman M., Goessens W.H., Endtz H.P., Boehm A.B., Faruque S.M. Environmental Spread of New Delhi Metal-lo-β-Lactamase-1-Producing Multidrug-Resistant Bacteria in Dhaka, Bangladesh. Appl. Environ. Microbiol. 2017;83:e00793-17. doi: 10.1128/AEM.00793-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Buchy P., Ascioglu S., Buisson Y., Datta S., Nissen M., Tambyah P.A., Vong S. Impact of vaccines on antimicrobial resistance. Int. J. Infect. Dis. 2020;90:188–196. doi: 10.1016/j.ijid.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 33.Thornber K., Verner-Jeffreys D., Hinchliffe S., Rahman M.M., Bass D., Tyler C.R. Evaluating antimicrobial resistance in the global shrimp industry. Rev. Aquac. 2020;12:966–986. doi: 10.1111/raq.12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aworh M.K., Kwaga J., Okolocha E., Harden L., Hull D., Hendriksen R.S., Thakur S. Extended-spectrum ß-lactamase-producing Escherichia coli among humans, chickens and poultry environments in Abuja, Nigeria. One Health Outlook. 2020;2:8. doi: 10.1186/s42522-020-00014-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van Boeckel T.P., Pires J., Silvester R., Zhao C., Song J., Criscuolo N.G., Gilbert M., Bonhoeffer S., Laxminarayan R. Global trends in antimicrobial resistance in animals in low- and middle-income countries. Science. 2019;365:1251. doi: 10.1126/science.aaw1944. [DOI] [PubMed] [Google Scholar]
- 36.Ogunleye O.O., Basu D., Mueller D., Sneddon J., Seaton R.A., Yinka-Ogunleye A.F., Wamboga J., Miljković N., Mwita J.C., Rwegerera G.M., et al. Response to the Novel Corona Virus (COVID-19) Pandemic Across Africa: Successes, Challenges, and Implications for the Future. Front. Pharm. 2020;11 doi: 10.3389/fphar.2020.01205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abbas K., Procter S.R., van Zandvoort K., Clark A., Funk S., Mengistu T., Hogan D., Dansereau E., Jit M., Flasche S. Routine childhood immunisation during the COVID-19 pandemic in Africa: A benefit-risk analysis of health benefits versus excess risk of SARS-CoV-2 infection. Lancet Glob. Health. 2020;8:e1264–e1272. doi: 10.1016/S2214-109X(20)30308-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Olorunsaiye C.Z., Yusuf K.K., Reinhart K., Salihu H.M. COVID-19 and Child Vaccination: A Systematic Approach to Closing the Immunization Gap. Int. J. Matern. Child Health AIDS. 2020;9:381–385. doi: 10.21106/ijma.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.WHO At Least 80 Million Children under One at Risk of Diseases such as Diphtheria, Measles and Polio as COVID-19 Disrupts Routine Vaccination Efforts, Warn Gavi, WHO and UNICEF. [(accessed on 8 May 2021)]; Available online: https://www.who.int/news/item/22-05-2020-at-least-80-million-children-under-one-at-risk-of-diseases-such-as-diphtheria-measles-and-polio-as-covid-19-disrupts-routine-vaccination-efforts-warn-gavi-who-and-unicef.
- 40.Atkins K.E., Flasche S. Vaccination to reduce antimicrobial resistance. Lancet Glob. Health. 2018;6:e252. doi: 10.1016/S2214-109X(18)30043-3. [DOI] [PubMed] [Google Scholar]
- 41.Rodríguez-Álvarez M., López-Vidal Y., Soto-Hernández J.L., Miranda-Novales M.G., Flores-Moreno K., de León-Rosales S.P. COVID-19: Clouds Over the Antimicrobial Resistance Landscape. Arch. Med. Res. 2021;52:123–126. doi: 10.1016/j.arcmed.2020.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Troisi M., Andreano E., Sala C., Kabanova A., Rappuoli R. Vaccines as remedy for antimicrobial resistance and emerging infections. Curr. Opin. Immunol. 2020;65:102–106. doi: 10.1016/j.coi.2020.09.003. [DOI] [PubMed] [Google Scholar]
- 43.Wilson S.L., Wiysonge C. Social media and vaccine hesitancy. BMJ Glob. Health. 2020;5:e004206. doi: 10.1136/bmjgh-2020-004206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Piedrahita-Valdés H., Piedrahita-Castillo D., Bermejo-Higuera J., Guillem-Saiz P., Bermejo-Higuera J.R., Guillem-Saiz J., Sicilia-Montalvo J.A., Machío-Regidor F. Vaccine Hesitancy on Social Media: Sentiment Analysis from June 2011 to April 2019. Vaccines. 2021;9:28. doi: 10.3390/vaccines9010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jarchow-MacDonald A.A., Burns R., Miller J., Kerr L., Willocks L.J. Keeping childhood immunisation rates stable during the COVID-19 pandemic. Lancet Infect. Dis. 2021;21:459–460. doi: 10.1016/S1473-3099(20)30991-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.UNA-SUS FIOCRUZ IN THE AIR: Covid-19 and Antibiotic Self-Medication: A Dangerous Combination. [(accessed on 8 May 2021)]; Available online: https://www.unasus.gov.br/noticia/fiocruz-no-ar-covid-19-e-a-automedicacao-de-antibioticos-uma-combinacao-perigosa.
- 47.Schueler P. Antibiotic Resistance and COVID-19. [(accessed on 7 May 2021)]; Available online: https://www.bio.fiocruz.br/index.php/br/noticias/1823-modernidade-e-sustentabilidade-no-centro-tecnologico-de-plataformas-vegetais.
- 48.Iwu C.J., Jordan P., Jaja I.F., Iwu C.D., Wiysonge C.S. Treatment of COVID-19: Implications for antimicrobial resistance in Africa. Pan Afr. Med. J. 2020;35 doi: 10.11604/pamj.supp.2020.35.2.23713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sefah I.A., Ogunleye O.O., Essah D.O., Opanga S.A., Butt N., Wamaitha A., Guantai A.N., Chikowe I., Khuluza F., Kibuule D., et al. Rapid Assessment of the Potential Paucity and Price Increases for Suggested Medicines and Protection Equipment for COVID-19 across Developing Countries with a Particular Focus on Africa and the Implications. Front. Pharm. 2021;11:588106. doi: 10.3389/fphar.2020.588106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ongole J.J., Rossouw T.M., Fourie P.B., Stoltz A.C., Hugo J., Marcus T.S. Sustaining essential healthcare in Africa during the COVID-19 pandemic. Int. J. Tuberc. Lung Dis. 2020;24:643–645. doi: 10.5588/ijtld.20.0214. [DOI] [PubMed] [Google Scholar]
- 51.Strathdee S.A., Davies S.C., Marcelin J.R. Confronting antimicrobial resistance beyond the COVID-19 pandemic and the 2020 US election. Lancet. 2020;396:1050–1053. doi: 10.1016/S0140-6736(20)32063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rawson T.M., Moore L.S.P., Zhu N., Ranganathan N., Skolimowska K., Gilchrist M., Satta G., Cooke G., Holmes A. Bacterial and Fungal Coinfection in Individuals with Coronavirus: A Rapid Review to Support COVID-19 Antimicrobial Prescribing. Clin. Infect. Dis. 2020;71:2459–2468. doi: 10.1093/cid/ciaa530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hsu J. How covid-19 is accelerating the threat of antimicrobial resistance. BMJ. 2020;369:m1983. doi: 10.1136/bmj.m1983. [DOI] [PubMed] [Google Scholar]
- 54.Nori P., Cowman K., Chen V., Bartash R., Szymczak W., Madaline T., Katiyar C.P., Jain R., Aldrich M., Weston G., et al. Bacterial and fungal coinfections in COVID-19 patients hospitalized during the New York City pandemic surge. Infect. Control Hosp. Epidemiol. 2021;42:84–88. doi: 10.1017/ice.2020.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Langford B.J., So M., Raybardhan S., Leung V., Soucy J.-P.R., Westwood D., Daneman N., MacFadden D.R. Antibiotic prescribing in patients with COVID-19: Rapid review and meta-analysis. Clin. Microbiol. Infect. 2021;27:520–531. doi: 10.1016/j.cmi.2020.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Interagency Coordination Group on Antimicrobial Resistance No Time to Wait: Securing the Future from Drug-Resistant Infections-Report to the Secretary-General of the United Nations. [(accessed on 8 May 2021)];2019 Apr; Available online: https://www.who.int/antimicrobial-resistance/interagency-coordination-group/IACG_final_report_EN.pdf?ua=1.
- 57.World Health Organisation Antimicrobial Resistance. [(accessed on 8 May 2021)];2018 Available online: http://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance.
- 58.WHO The Role of Pharmacist in Encouraging Prudent Use of Antibiotics and Averting Antimicrobial Resistance: A Review of Policy and Experience. [(accessed on 7 May 2021)];2014 Available online: http://www.euro.who.int/__data/assets/pdf_file/0006/262815/The-role-of-pharmacist-in-encouraging-prudent-use-of-antibiotics-and-averting-antimicrobial-resistance-a-review-of-policy-and-experience-Eng.pdf?ua=1.
- 59.Federal Ministries of Agriculture, Rural Development, Environment and Health, Abuja, Nigeria National Action Plan for Antimicrobial Resistance, 2017–2022. [(accessed on 8 May 2021)]; Available online: https://ncdc.gov.ng/themes/common/docs/protocols/77_1511368219.pdf.
- 60.Saleem Z., Hassali M.A., Hashmi F.K. Pakistan’s national action plan for antimicrobial resistance: Translating ideas into reality. Lancet Infect. Dis. 2018;18:1066–1067. doi: 10.1016/S1473-3099(18)30516-4. [DOI] [PubMed] [Google Scholar]
- 61.Fürst J., Čižman M., Mrak J., Kos D., Campbell S., Coenen S., Gustafsson L.L., Fürst L., Godman B. The influence of a sustained multifaceted approach to improve antibiotic prescribing in Slovenia during the past decade: Findings and implications. Expert Rev. Anti-Infect. Ther. 2014;13:279–289. doi: 10.1586/14787210.2015.990381. [DOI] [PubMed] [Google Scholar]
- 62.Abilova V., Kurdi A., Godman B. Ongoing initiatives in Azerbaijan to improve the use of antibiotics; findings and implications. Expert Rev. Anti-Infect. Ther. 2018;16:77–84. doi: 10.1080/14787210.2018.1417835. [DOI] [PubMed] [Google Scholar]
- 63.Engler D., Meyer J.C., Schellack N., Kurdi A., Godman B. Compliance with South Africa’s Antimicrobial Resistance National Strategy Framework: Are we there yet? J. Chemother. 2021;33:21–31. doi: 10.1080/1120009X.2020.1789389. [DOI] [PubMed] [Google Scholar]
- 64.Godman B., Haque M., McKimm J., Abu Bakar M., Sneddon J., Wale J., Campbell S., Martin A., Hoxha I., Abilova V., et al. Ongoing strategies to improve the management of upper respiratory tract infections and reduce inappropriate antibiotic use particularly among lower and middle-income countries: Findings and implications for the future. Curr. Med. Res. Opin. 2019;36:301–327. doi: 10.1080/03007995.2019.1700947. [DOI] [PubMed] [Google Scholar]
- 65.Government of India National Action Plan Antimicrobial Resistance (NAP-AMR) 2017–2021. [(accessed on 7 May 2021)]; Available online: https://rr-asia.oie.int/wp-content/uploads/2020/03/india_national-action-plan-on-amr-india.pdf.
- 66.Bao L., Peng R., Wang Y., Ma R., Ren X., Meng W., Sun F., Fang J., Chen P., Wang Y., et al. Significant Reduction of Antibiotic Consumption and Patients’ Costs after an Action Plan in China, 2010–2014. PLoS ONE. 2015;10:e0118868. doi: 10.1371/journal.pone.0118868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Versporten A., Bolokhovets G., Ghazaryan L., Abilova V., Pyshnik G., Spasojevic T., Korinteli I., Raka L., Kambaralieva B., Cizmovic L., et al. Antibiotic use in eastern Europe: A cross-national database study in coordination with the WHO Regional Office for Europe. Lancet Infect. Dis. 2014;14:381–387. doi: 10.1016/S1473-3099(14)70071-4. [DOI] [PubMed] [Google Scholar]
- 68.Brazilian Health Regulatory Agency National Program for the Prevention and Control of Healthcare Associated Infections (2016–2020) [(accessed on 8 May 2021)]; Available online: https://www.gov.br/anvisa/pt-br/centraisdeconteudo/publicacoes/servicosdesaude/publicacoes/national-program-for-the-prevention-and-control-of-healthcare-associated-infections-2016-2020.
- 69.WHO Global Action Plan on Antimicrobial Resistance. [(accessed on 7 May 2021)];2015 Available online: https://apps.who.int/iris/bitstream/handle/10665/193736/9789241509763_eng.pdf?sequence=1.
- 70.Ghana Ministry of Health, Ministry of Food and Agriculture, Ministry of Environment, Science, Technology and Innovation, Ministry of Fisheries and Aquaculture Development Ghana National Action Plan for Antimicrobial Use and Resistance. 2017–2021. [(accessed on 7 May 2021)]; Available online: http://www.moh.gov.gh/wp-content/uploads/2018/04/NAP_FINAL_PDF_A4_19.03.2018-SIGNED-1.pdf.
- 71.Mendelson M., Matsoso M.P. The South African Antimicrobial Resistance Strategy Framework. AMR Control. 2015:54–61. [Google Scholar]
- 72.Republic of Kenya National Action Plan on Prevention and Containment of Antimicrobial Re-Sistance, 2017–2022. [(accessed on 10 May 2021)]; Available online: https://www.afro.who.int/publications/national-action-plan-prevention-and-containment-antimicrobial-resistance-2017-2022.
- 73.Ministry of Health and Family Welfare (MoHFW), Government of Bangladesh National Action Plan: Antimicrobial Resistance Containment in Bangladesh 2017-’22. [(accessed on 7 May 2021)]; Available online: https://www.flemingfund.org/wp-content/uploads/d3379eafad36f597500cb07c21771ae3.pdf.
- 74.Munkholm L., Rubin O. The global governance of antimicrobial resistance: A cross-country study of alignment between the global action plan and national action plans. Glob. Health. 2020;16:109. doi: 10.1186/s12992-020-00639-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zaney G.D. Ghana’s National Action Plan on AMR Implementation on Course—But It Requires Support from All. [(accessed on 8 May 2021)]; Available online: https://allafrica.com/stories/201908270183.html.
- 76.Sangeda R.Z., Kibona J., Munishi C., Arabi F., Manyanga V.P., Mwambete K.D., Horumpende P.G. Assessment of Implementation of Antimicrobial Resistance Surveillance and Antimicrobial Stewardship Programs in Tanzanian Health Facilities a Year after Launch of the National Action Plan. Front. Public Health. 2020;8:454. doi: 10.3389/fpubh.2020.00454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bajehson M., Musa B.M., Gidado M., Nsa B., Sani U., Habibu A.T., Aliyu I., Hussaini T., Yusuf A., Gida Y. Determinants of mortality among patients with drug-resistant tuberculosis in northern Nigeria. PLoS ONE. 2019;14:e0225165. doi: 10.1371/journal.pone.0225165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Akram J., Khan A.S., Khan H.A., Gilani S.A., Akram S.J., Ahmad F.J., Mehboob R. Extensively Drug-Resistant (XDR) Typhoid: Evolution, Prevention, and Its Management. BioMed Res. Int. 2020;2020:6432580. doi: 10.1155/2020/6432580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Kurbatova E.V., Taylor A., Gammino V.M., Bayona J., Becerra M., Danilovitz M., Falzon D., Gelmanova I., Keshavjee S., Leimane V., et al. Predictors of poor outcomes among patients treated for multidrug-resistant tuberculosis at DOTS-plus projects. Tuberculosis. 2012;92:397–403. doi: 10.1016/j.tube.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ali M.H., Alrasheedy A.A., Kibuule D., Godman B., Hassali M.A., Ali H.M.H. Assessment of multidrug-resistant tuberculosis (MDR-TB) treatment outcomes in Sudan; findings and implications. Expert Rev. Anti-Infect. Ther. 2019;17:927–937. doi: 10.1080/14787210.2019.1689818. [DOI] [PubMed] [Google Scholar]
- 81.Bojanić L., Markovic-Pekovic V., Skrbic R., Stojakovic N., Ðermanović M., Bojanić J., Fürst J., Kurdi A.B., Godman B. Recent Initiatives in the Republic of Srpska to Enhance Appropriate Use of Antibiotics in Ambulatory Care; Their Influence and Implications. Front. Pharm. 2018;9 doi: 10.3389/fphar.2018.00442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Wojkowska-Mach J., Godman B., Glassman A., Kurdi A., Pilc A., Rozanska A., Skoczyński S., Wałaszek M., Bochenek T. Antibiotic consumption and antimicrobial resistance in Poland; findings and implications. Antimicrob. Resist. Infect. Control. 2018;7:136. doi: 10.1186/s13756-018-0428-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Saleem Z., Hassali M.A., Godman B., Hashmi F.K., Saleem F. A multicenter point prevalence survey of healthcare-associated infections in Pakistan: Findings and implications. Am. J. Infect. Control. 2019;47:421–424. doi: 10.1016/j.ajic.2018.09.025. [DOI] [PubMed] [Google Scholar]
- 84.Mwita J.C., Ogunleye O.O., Olalekan A., Kalungia A.C., Kurdi A., Saleem Z., Sneddon J., Godman B. Key Issues Surrounding Appropriate Antibiotic Use for Prevention of Surgical Site Infections in Low- and Middle-Income Countries: A Narrative Review and the Implications. Int. J. Gen. Med. 2021;14:515–530. doi: 10.2147/IJGM.S253216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Haque M., McKimm J., Godman B., Abu Bakar M., Sartelli M. Initiatives to reduce postoperative surgical site infections of the head and neck cancer surgery with a special emphasis on developing countries. Expert Rev. Anticancer. Ther. 2018;19:81–92. doi: 10.1080/14737140.2019.1544497. [DOI] [PubMed] [Google Scholar]
- 86.Nathwani D., Varghese D., Stephens J., Ansari W., Martin S., Charbonneau C. Value of hospital antimicrobial stewardship programs [ASPs]: A systematic review. Antimicrob. Resist. Infect. Control. 2019;8:35. doi: 10.1186/s13756-019-0471-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Akpan M.R., Isemin N.U., Udoh A.E., Ashiru-Oredope D. Implementation of antimicrobial stewardship programmes in African countries: A systematic literature review. J. Glob. Antimicrob. Resist. 2020;22:317–324. doi: 10.1016/j.jgar.2020.03.009. [DOI] [PubMed] [Google Scholar]
- 88.Anand Paramadhas B.D., Tiroyakgosi C., Mpinda-Joseph P., Morokotso M., Matome M., Sinkala F., Gaolebe M., Malone B., Molosiwa E., Shanmugam M.G., et al. Point prevalence study of antimicrobial use among hospitals across Botswana; findings and implications. Expert Rev. Anti-Infect. Ther. 2019;17:535–546. doi: 10.1080/14787210.2019.1629288. [DOI] [PubMed] [Google Scholar]
- 89.Cai Y., Shek P.Y., Teo I., Tang S.S., Lee W., Liew Y.X., Chlebicki P., Kwa A.L. A multidisciplinary antimicrobial stewardship programme safely decreases the duration of broad-spectrum antibiotic prescription in Singaporean adult renal patients. Int. J. Antimicrob. Agents. 2016;47:91–96. doi: 10.1016/j.ijantimicag.2015.10.021. [DOI] [PubMed] [Google Scholar]
- 90.Tang S.J., Gupta R., Lee J.I., Majid A.M., Patel P., Efird L., Loo A., Mazur S., Calfee D.P., Archambault A., et al. Impact of Hospitalist-Led Interdisciplinary Antimicrobial Stewardship Interventions at an Academic Medical Center. Jt. Comm. J. Qual. Patient Saf. 2019;45:207–216. doi: 10.1016/j.jcjq.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 91.Schuts E.C., Hulscher M., Mouton J.W., Verduin C.M., Stuart J., Overdiek H., van der Linden P.D., Natsch S., Hertogh C.M.P.M., Wolfs T.F.W., et al. Current evidence on hospital antimicrobial stewardship objectives: A systematic review and meta-analysis. Lancet Infect. Dis. 2016;16:847–856. doi: 10.1016/S1473-3099(16)00065-7. [DOI] [PubMed] [Google Scholar]
- 92.Shirazi O.U., Ab Rahman N.S., Zin C.S. A Narrative Review of Antimicrobial Stewardship Interventions within In-Patient Settings and Resultant Patient Outcomes. J. Pharm. Bioallied. Sci. 2020;12:369–380. doi: 10.4103/jpbs.JPBS_311_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Satterfield J., Miesner A.R., Percival K.M. The role of education in antimicrobial stewardship. J. Hosp. Infect. 2020;105:130–141. doi: 10.1016/j.jhin.2020.03.028. [DOI] [PubMed] [Google Scholar]
- 94.Seni J., Mapunjo S.G., Wittenauer R., Valimba R., Stergachis A., Werth B.J., Saitoti S., Mhadu N.H., Lusaya E., Konduri N. Antimicrobial use across six referral hospitals in Tanzania: A point prevalence survey. BMJ Open. 2020;10:e042819. doi: 10.1136/bmjopen-2020-042819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Álvarez-Marín R., López-Cerero L., Guerrero-Sánchez F., Palop-Borras B., Rojo-Martín M.D., Ruiz-Sancho A., Herrero-Rodríguez C., García M.V., Lazo-Torres A.M., López I., et al. Do specific antimicrobial stewardship interventions have an impact on carbapenem resistance in Gram-negative bacilli? A multicentre quasi-experimental ecological study: Time-trend analysis and characterization of carbapenemases. J. Antimicrob. Chemother. 2021 doi: 10.1093/jac/dkab073. [DOI] [PubMed] [Google Scholar]
- 96.López-Viñau T., Peñalva G., García-Martínez L., Castón J.J., Muñoz-Rosa M., Cano Á., Recio M., Cisneros J.M., Pérez-Nadales E., Rumbao Aguirre J., et al. Impact of an Antimicrobial Stewardship Program on the Incidence of Carbapenem Resistant Gram-Negative Bacilli: An Interrupted Time-Series Analysis. Antibiotics. 2021;10:586. doi: 10.3390/antibiotics10050586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cox J.A., Vlieghe E., Mendelson M., Wertheim H., Ndegwa L., Villegas M.V., Gould I., Hara G.L. Antibiotic stewardship in low- and middle-income countries: The same but different? Clin. Microbiol. Infect. 2017;23:812–818. doi: 10.1016/j.cmi.2017.07.010. [DOI] [PubMed] [Google Scholar]
- 98.Brink A.J., Messina A.P., Feldman C., Richards G.A., Becker P.J., Goff D.A., Bauer K.A., Nathwani D., Bergh D.V.D. Antimicrobial stewardship across 47 South African hospitals: An implementation study. Lancet Infect. Dis. 2016;16:1017–1025. doi: 10.1016/S1473-3099(16)30012-3. [DOI] [PubMed] [Google Scholar]
- 99.Mukokinya M.M.A., Opanga S., Oluka M., Godman B. Dispensing of antimicrobials in Kenya: A cross-sectional pilot study and its implications. J. Res. Pharm. Pract. 2018;7:77. doi: 10.4103/jrpp.jrpp_17_88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jacobs T.G., Robertson J., van den Ham H.A., Iwamoto K., Pedersen H.B., Mantel-Teeuwisse A.K. Assessing the impact of law enforcement to reduce over-the-counter (OTC) sales of antibiotics in low- and middle-income countries; a systematic literature review. BMC Health Serv. Res. 2019;19:536. doi: 10.1186/s12913-019-4359-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Olaoye O., Tuck C., Khor W.P., McMenamin R., Hudson L., Northall M., Panford-Quainoo E., Asima D.M., Ashiru-Oredope D. Improving Access to Antimicrobial Prescribing Guidelines in 4 African Countries: Development and Pilot Implementation of an App and Cross-Sectional Assessment of Attitudes and Behaviour Survey of Healthcare Workers and Patients. Antibiotics. 2020;9:555. doi: 10.3390/antibiotics9090555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gasson J., Blockman M., Willems B. Antibiotic prescribing practice and adherence to guidelines in primary care in the Cape Town Metro District, South Africa. S. Afr. Med. J. 2018;108:304–310. doi: 10.7196/SAMJ.2018.v108i4.12564. [DOI] [PubMed] [Google Scholar]
- 103.Elias C., Moja L., Mertz D., Loeb M., Forte G., Magrini N. Guideline recommendations and antimicrobial resistance: The need for a change. BMJ Open. 2017;7:e016264. doi: 10.1136/bmjopen-2017-016264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Saleem Z., Godman B., Hassali M.A., Hashmi F.K., Azhar F., Rehman I.U. Point prevalence surveys of health-care-associated infections: A systematic review. Pathog. Glob. Health. 2019;113:191–205. doi: 10.1080/20477724.2019.1632070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Saleem Z., Hassali M.A., Godman B., Versporten A., Hashmi F.K., Saeed H., Saleem F., Salman M., Rehman I.U., Khan T.M. Point prevalence surveys of antimicrobial use: A systematic review and the implications. Expert Rev. Anti-Infect. Ther. 2020;18:897–910. doi: 10.1080/14787210.2020.1767593. [DOI] [PubMed] [Google Scholar]
- 106.Rappuoli R., De Gregorio E., Del Giudice G., Phogat S., Pecetta S., Pizza M., Hanon E. Vaccinology in the post-COVID-19 era. Proc. Natl. Acad. Sci. USA. 2021;118:e2020368118. doi: 10.1073/pnas.2020368118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Batura N., Cuevas C., Khan M., Wiseman V. How effective and cost-effective are behaviour change interventions in improving the prescription and use of antibiotics in low-income and middle-income countries? A protocol for a systematic review. BMJ Open. 2018;8:e021517. doi: 10.1136/bmjopen-2018-021517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cooper L., Sneddon J., Afriyie D.K., Sefah I.A., Kurdi A., Godman B., Seaton R.A. Supporting global antimicrobial stewardship: Antibiotic prophylaxis for the prevention of surgical site infection in low- and middle-income countries (LMICs): A scoping review and meta-analysis. JAC-Antimicrob. Resist. 2020;2 doi: 10.1093/jacamr/dlaa070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ocan M., Obuku E.A., Bwanga F., Akena D., Richard S., Ogwal-Okeng J., Obua C. Household antimicrobial self-medication: A systematic review and meta-analysis of the burden, risk factors and outcomes in developing countries. BMC Public Health. 2015;15:742. doi: 10.1186/s12889-015-2109-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.da Silva A.A., de Almeida Dias D.A., Marques A.F., di Biase C.B., Murni I.K., Dramowski A., Sharland M., Huebner J., Zingg W. Role of antimicrobial stewardship programmes in children: A systematic review. J. Hosp. Infect. 2018;99:117–123. doi: 10.1016/j.jhin.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 111.Huebner C., Flessa S., Huebner N.O. The economic impact of antimicrobial stewardship programmes in hospitals: A systematic literature review. J. Hosp. Infect. 2019;102:369–376. doi: 10.1016/j.jhin.2019.03.002. [DOI] [PubMed] [Google Scholar]
- 112.Karanika S., Paudel S., Grigoras C., Kalbasi A., Mylonakis E. Systematic Review and Meta-analysis of Clinical and Economic Outcomes from the Implementation of Hospital-Based Antimicrobial Stewardship Programs. Antimicrob. Agents Chemother. 2016;60:4840–4852. doi: 10.1128/AAC.00825-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Holloway K.A., Rosella L., Henry D. The Impact of WHO Essential Medicines Policies on Inappropriate Use of Antibiotics. PLoS ONE. 2016;11:e0152020. doi: 10.1371/journal.pone.0152020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Md Rezal R.S., Hassali M.A., Alrasheedy A.A., Saleem F., Md Yusof F.A., Godman B. Physicians’ knowledge, perceptions and behaviour towards antibiotic prescribing: A systematic review of the literature. Expert Rev. Anti-Infect. Ther. 2015;13:665–680. doi: 10.1586/14787210.2015.1025057. [DOI] [PubMed] [Google Scholar]
- 115.Dar O.A., Hasan R., Schlundt J., Harbarth S., Caleo G., Dar F.K., Littmann J., Rweyemamu M., Buckley E.J., Shahid M., et al. Exploring the evidence base for national and regional policy interventions to combat resistance. Lancet. 2016;387:285–295. doi: 10.1016/S0140-6736(15)00520-6. [DOI] [PubMed] [Google Scholar]
- 116.McDonagh M.S., Peterson K., Winthrop K., Cantor A., Lazur B.H., Buckley D.I. Interventions to reduce inappropriate prescribing of antibiotics for acute respiratory tract infections: Summary and update of a systematic review. J. Int. Med. Res. 2018;46:3337–3357. doi: 10.1177/0300060518782519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Dyar O.J., Beovic B., Vlahovic-Palcevski V., Verheij T., Pulcini C. How can we improve antibiotic prescribing in primary care? Expert Rev. Anti-Infect. Ther. 2016;14:403–413. doi: 10.1586/14787210.2016.1151353. [DOI] [PubMed] [Google Scholar]
- 118.Auta A., Hadi M.A., Oga E., Adewuyi E.O., Abdu-Aguye S.N., Adeloye D., Strickland-Hodge B., Morgan D.J. Global access to antibiotics without prescription in community pharmacies: A systematic review and meta-analysis. J. Infect. 2019;78:8–18. doi: 10.1016/j.jinf.2018.07.001. [DOI] [PubMed] [Google Scholar]
- 119.Köchling A., Löffler C., Reinsch S., Hornung A., Böhmer F., Altiner A., Chenot J.F. Reduction of antibiotic prescriptions for acute respiratory tract infections in primary care: A systematic review. Implement. Sci. 2018;13:47. doi: 10.1186/s13012-018-0732-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.McKay R., Mah A., Law M.R., McGrail K., Patrick D.M. Systematic Review of Factors Associated with Antibiotic Prescribing for Respiratory Tract Infections. Antimicrob. Agents Chemother. 2016;60:4106–4118. doi: 10.1128/AAC.00209-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Roque F., Herdeiro M.T., Soares S., Teixeira Rodrigues A., Breitenfeld L., Figueiras A. Educational interventions to improve prescription and dispensing of antibiotics: A systematic review. BMC Public Health. 2014;14:1276. doi: 10.1186/1471-2458-14-1276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Teixeira Rodrigues A., Roque F., Falcao A., Figueiras A., Herdeiro M.T. Understanding physician antibiotic prescribing behaviour: A systematic review of qualitative studies. Int. J. Antimicrob. Agents. 2013;41:203–212. doi: 10.1016/j.ijantimicag.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 123.Sakeena M.H.F., Bennett A.A., McLachlan A.J. Non-prescription sales of antimicrobial agents at community pharmacies in developing countries: A systematic review. Int. J. Antimicrob. Agents. 2018;52:771–782. doi: 10.1016/j.ijantimicag.2018.09.022. [DOI] [PubMed] [Google Scholar]
- 124.Nepal G., Bhatta S. Self-medication with Antibiotics in WHO Southeast Asian Region: A Systematic Review. Cureus. 2018;10:e2428. doi: 10.7759/cureus.2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zanichelli V., Tebano G., Gyssens I.C., Vlahović-Palčevski V., Monnier A.A., Benic M.S., Harbarth S., Hulscher M., Pulcini C., Huttner B. Patient-related determinants of antibiotic use: A systematic review. Clin. Microbiol. Infect. 2019;25:48–53. doi: 10.1016/j.cmi.2018.04.031. [DOI] [PubMed] [Google Scholar]
- 126.Servia-Dopazo M., Figueiras A. Determinants of antibiotic dispensing without prescription: A systematic review. J. Antimicrob. Chemother. 2018;73:3244–3253. doi: 10.1093/jac/dky319. [DOI] [PubMed] [Google Scholar]
- 127.Lescure D., Paget J., Schellevis F., Van Dijk L. Determinants of Self-Medication with Antibiotics in European and Anglo-Saxon Countries: A Systematic Review of the Literature. Front. Public Health. 2018;6:370. doi: 10.3389/fpubh.2018.00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Saharman Y.R., Karuniawati A., Severin J.A., Verbrugh H.A. Infections and antimicrobial resistance in intensive care units in lower-middle income countries: A scoping review. Antimicrob. Resist. Infect. Control. 2021;10:22. doi: 10.1186/s13756-020-00871-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Torres N.F., Chibi B., Middleton L.E., Solomon V.P., Mashamba-Thompson T.P. Evidence of factors influencing self-medication with antibiotics in low and middle-income countries: A systematic scoping review. Public Health. 2019;168:92–101. doi: 10.1016/j.puhe.2018.11.018. [DOI] [PubMed] [Google Scholar]
- 130.Irek E.O., Amupitan A.A., Obadare T.O., Aboderin A.O. A systematic review of healthcare-associated infections in Africa: An antimicrobial resistance perspective. Afr. J. Lab. Med. 2018;7:9. doi: 10.4102/ajlm.v7i2.796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Badia J.M., Casey A.L., Petrosillo N., Hudson P.M., Mitchell S.A., Crosby C. Impact of surgical site infection on healthcare costs and patient outcomes: A systematic review in six European countries. J. Hosp. Infect. 2017;96:1–15. doi: 10.1016/j.jhin.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 132.Schreiber P.W., Sax H., Wolfensberger A., Clack L., Kuster S.P. Swissnoso the preventable proportion of healthcare-associated infections 2005–2016: Systematic review and meta-analysis. Infect. Control Hosp. Epidemiol. 2018;39:1277–1295. doi: 10.1017/ice.2018.183. [DOI] [PubMed] [Google Scholar]
- 133.Alhomoud F., Aljamea Z., Almahasnah R., Alkhalifah K., Basalelah L., Alhomoud F.K. Self-medication and self-prescription with antibiotics in the Middle East-do they really happen? A systematic review of the prevalence, possible reasons, and outcomes. Int. J. Infect. Dis. 2017;57:3–12. doi: 10.1016/j.ijid.2017.01.014. [DOI] [PubMed] [Google Scholar]
- 134.May L.S., Quirós A.M., Ten Oever J., Hoogerwerf J.J., Schoffelen T., Schouten J.A. Antimicrobial stewardship in the emergency department: Characteristics and evidence for effectiveness of interventions. Clin. Microbiol. Infect. 2021;27:204–209. doi: 10.1016/j.cmi.2020.10.028. [DOI] [PubMed] [Google Scholar]
- 135.Kalungia A., Godman B. Implications of non-prescription antibiotic sales in China. Lancet Infect. Dis. 2019;19:1272–1273. doi: 10.1016/S1473-3099(19)30408-6. [DOI] [PubMed] [Google Scholar]
- 136.Kalungia A.C., Burger J., Godman B., Costa J.D.O., Simuwelu C. Non-prescription sale and dispensing of antibiotics in community pharmacies in Zambia. Expert Rev. Anti-Infect. Ther. 2016;14:1215–1223. doi: 10.1080/14787210.2016.1227702. [DOI] [PubMed] [Google Scholar]
- 137.Godman B., Fadare J., Kibuule D., Irawati L., Mubita M., Ogunleye O., Oluka M., Paramadhas B.D.A., Costa J.D.O., De Lemos L.L.P., et al. Drug Resistance in Bacteria, Fungi, Malaria, and Cancer. Volume 34. Springer Science and Business Media; Berlin/Heidelberg, Germany: 2017. Initiatives Across Countries to Reduce Antibiotic Utilisation and Resistance Patterns: Impact and Implications; pp. 539–576. [Google Scholar]
- 138.Godman B., McCabe H., DLeong T., Mueller D., Martin A.P., Hoxha I., Mwita J.C., Rwegerera G.M., Massele A., Costa J.D., et al. Fixed dose drug combinations—Are they pharmacoeconomically sound? Findings and impli-cations especially for lower- and middle-income countries. Expert Rev. Pharm. Outcomes Res. 2020;20:1–26. doi: 10.1080/14737167.2020.1734456. [DOI] [PubMed] [Google Scholar]
- 139.Godman B., Malmstrom R.E., Diogene E., Jayathissa S., McTaggart S., Cars T., Alvarez-Madrazo S., Baumgärtel C., Brzezinska A., Bucsics A., et al. Dabigatran—A continuing exemplar case history demonstrating the need for comprehensive models to optimize the utilization of new drugs. Front. Pharmacol. 2014;5:109. doi: 10.3389/fphar.2014.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Godman B., Hill A., Simoens S., Selke G., Selke Krulichová I., Zampirolli Dias C., Martin A.P., Oortwijn W., Timoney A., Gustafsson L.L., et al. Potential approaches for the pricing of cancer medicines across Europe to enhance the sustainability of healthcare systems and the implications. Expert Rev. Pharm. Outcomes Res. 2021:1–14. doi: 10.1080/14737167.2021.1884546. [DOI] [PubMed] [Google Scholar]
- 141.Dias C.Z., Godman B., Gargano L.P., Azevedo P.S., Garcia M.M., Cazarim M.S., Pantuzza L.L., Ribeiro-Junior N.G., Pereira A.L., Borin M.C., et al. Integrative Review of Managed Entry Agreements: Chances and Limitations. PharmacoEconomics. 2020;38:1165–1185. doi: 10.1007/s40273-020-00943-1. [DOI] [PubMed] [Google Scholar]
- 142.Godman B., Bucsics A., Vella Bonanno P., Oortwijn W., Rothe C.C., Ferrario A., Bosselli S., Hill A., Martin A., Simoens S., et al. Barriers for Access to New Medicines: Searching for the Balance between Rising Costs and Limited Budgets. Front. Public Health. 2018;6:328. doi: 10.3389/fpubh.2018.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Moorkens E., Vulto A.G., Huys I., Dylst P., Godman B., Keuerleber S., Claus B., Dimitrova M., Petrova G., Sović-Brkičić L., et al. Policies for biosimilar uptake in Europe: An overview. PLoS ONE. 2017;12:e0190147. doi: 10.1371/journal.pone.0190147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ermisch M., Bucsics A., Vella Bonanno P., Arickx F., Bybau A., Bochenek T., Van de Casteele M., Diogene E., Fürst J., Garuolienė K., et al. Payers’ Views of the Changes Arising through the Possible Adoption of Adaptive Pathways. Front. Pharm. 2016;7 doi: 10.3389/fphar.2016.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bochenek T., Abilova V., Alkan A., Asanin B., de Miguel Beriain I., Besovic Z., Bonanno P.V., Bucsics A., Davidescu M., De Weerdt E., et al. Systemic Measures and Legislative and Organizational Frameworks Aimed at Preventing or Mitigating Drug Shortages in 28 European and Western Asian Countries. Front. Pharm. 2018;8:942. doi: 10.3389/fphar.2017.00942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Godman B., Grobler C., Van-De-Lisle M., Wale J., Barbosa W.B., Massele A., Opondo P., Petrova G., Tachkov K., Sefah I., et al. Pharmacotherapeutic interventions for bipolar disorder type II: Addressing multiple symptoms and approaches with a particular emphasis on strategies in lower and middle-income countries. Expert Opin. Pharmacother. 2019;20:2237–2255. doi: 10.1080/14656566.2019.1684473. [DOI] [PubMed] [Google Scholar]
- 147.Godman B., Wettermark B., Van Woerkom M., Fraeyman J., Alvarez-Madrazo S., Berg C., Bishop I., Bucsics A., Campbell S., Finlayson A.E., et al. Multiple policies to enhance prescribing efficiency for established medicines in Europe with a particular focus on demand-side measures: Findings and future implications. Front. Pharm. 2014;5:106. doi: 10.3389/fphar.2014.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.World Bank World Bank Country and Lending Groups—Country Classifictions. [(accessed on 8 May 2021)]; Available online: https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups.
- 149.Cameron A., Ewen M., Ross-Degnan D., Ball D., Laing R. Medicine prices, availability, and affordability in 36 developing and middle-income countries: A secondary analysis. Lancet. 2009;373:240–249. doi: 10.1016/S0140-6736(08)61762-6. [DOI] [PubMed] [Google Scholar]
- 150.Ofori-Asenso R., Agyeman A.A. Irrational Use of Medicines—A Summary of Key Concepts. Pharmacy. 2016;4:35. doi: 10.3390/pharmacy4040035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Aregbeshola B.S., Khan S.M. Out-of-Pocket Payments, Catastrophic Health Expenditure and Poverty among Households in Nigeria. Int. J. Health Policy Manag. 2018;7:798–806. doi: 10.15171/ijhpm.2018.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Khan J.A.M., Ahmed S., Evans T.G. Catastrophic healthcare expenditure and poverty related to out-of-pocket payments for healthcare in Bangladesh—An estimation of financial risk protection of universal health coverage. Health Policy Plan. 2017;32:1102–1110. doi: 10.1093/heapol/czx048. [DOI] [PubMed] [Google Scholar]
- 153.Godman B., Haque M., Islam S., Iqbal S., Urmi U.L., Kamal Z.M., Shuvo S.A., Rahman A., Kamal M., Haque M., et al. Rapid Assessment of Price Instability and Paucity of Medicines and Protection for COVID-19 across Asia: Findings and Public Health Implications for the Future. Front. Public Health. 2020;8:585832. doi: 10.3389/fpubh.2020.585832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Murphy A., Palafox B., Walli-Attaei M., Powell-Jackson T., Rangarajan S., Alhabib K.F., Avezum A.J., Calik K.B.T., Chifamba J., Choudhury T., et al. The household economic burden of non-communicable diseases in 18 countries. BMJ Glob. Health. 2020;5:e002040. doi: 10.1136/bmjgh-2019-002040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rezal R.S., Hassali M.A., Alrasheedy A.A., Saleem F., Aryani Md Yusof F., Kamal M., Din R.M., Godman B. Prescribing patterns for upper respiratory tract infections: A prescription-review of primary care practice in Kedah, Malaysia, and the implications. Expert Rev. Anti-Infect. Ther. 2015;13:1547–1556. doi: 10.1586/14787210.2015.1085303. [DOI] [PubMed] [Google Scholar]
- 156.Chowdhury M., Stewart Williams J., Wertheim H., Khan W.A., Matin A., Kinsman J. Rural community perceptions of antibiotic access and understanding of antimicrobial resistance: Qualitative evidence from the Health and Demographic Surveillance System site in Matlab, Bangladesh. Glob. Health Action. 2019;12(Suppl. 1):1824383. doi: 10.1080/16549716.2020.1824383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Markovic-Pekovic V., Grubiša N., Burger J., Bojanić L., Godman B. Initiatives to reduce nonprescription sales and dispensing of antibiotics: Findings and implications. J. Res. Pharm. Pract. 2017;6:120. doi: 10.4103/jrpp.jrpp_17_12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Nashilongo M.M., Singu B., Kalemeera F., Mubita M., Naikaku E., Baker A., Ferrario A., Godman B., Achieng L., Kibuule D. Assessing Adherence to Antihypertensive Therapy in Primary Health Care in Namibia: Findings and Implications. Cardiovasc. Drugs. 2017;31:565–578. doi: 10.1007/s10557-017-6756-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Saleem Z., Hassali M.A., Hashmi F.K., Godman B., Saleem F. Antimicrobial dispensing practices and determinants of antimicrobial resistance: A qualitative study among community pharmacists in Pakistan. Fam. Med. Community Health. 2019;7:e000138. doi: 10.1136/fmch-2019-000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chang J., Xu S., Zhu S., Li Z., Yu J., Zhang Y., Zu J., Fang Y., Ross-Degnan D. Assessment of non-prescription antibiotic dispensing at community pharmacies in China with simulated clients: A mixed cross-sectional and longitudinal study. Lancet Infect. Dis. 2019;19:1345–1354. doi: 10.1016/S1473-3099(19)30324-X. [DOI] [PubMed] [Google Scholar]
- 161.WHO Community Pharmacists Are Key Players in COVID-19 Response and Must Stay Up-to-Date on Guidance. [(accessed on 8 May 2020)];2020 Available online: https://www.euro.who.int/en/health-topics/health-emergencies/coronavirus-covid-19/news/news/2020/5/community-pharmacists-are-key-players-in-covid-19-response-and-must-stay-up-to-date-on-guidance.
- 162.Hoxha I., Malaj A., Kraja B., Bino S., Oluka M., Markovic-Pekovic V., Godman B. Are pharmacists’ good knowledge and awareness on antibiotics taken for granted? The situation in Albania and future implications across countries. J. Glob. Antimicrob. Resist. 2018;13:240–245. doi: 10.1016/j.jgar.2018.01.019. [DOI] [PubMed] [Google Scholar]
- 163.Darj E., Newaz M.S., Zaman M.H. Pharmacists’ perception of their challenges at work, focusing on antimicrobial resistance: A qualitative study from Bangladesh. Glob. Health Action. 2019;12(Suppl. 1):1735126. doi: 10.1080/16549716.2020.1735126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Waseem H., Ali J., Sarwar F., Khan A., Rehman H.S.U., Choudri M., Arif N., Subhan M., Saleem A.R., Jamal A., et al. Assessment of knowledge and attitude trends towards antimicrobial resistance (AMR) among the community members, pharmacists/pharmacy owners and physicians in district Sialkot, Pakistan. Antimicrob. Resist. Infect. Control. 2019;8:67. doi: 10.1186/s13756-019-0517-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wettermark B., Godman B., Jacobsson B., Haaijer-Ruskamp F.M. Soft regulations in pharmaceutical policy making: An overview of current approaches and their consequences. Appl. Health Econ. Health Policy. 2009;7:137–147. doi: 10.1007/BF03256147. [DOI] [PubMed] [Google Scholar]
- 166.Godman B. Health authority activities to enhance the quality and efficiency of medicine use and their impact. Adv. Hum. Biol. 2021;11:11–16. [Google Scholar]
- 167.Niaz Q., Godman B., Massele A., Campbell S., Kurdi A., Kagoya H.R., Kibuule D. Validity of World Health Organisation prescribing indicators in Namibia’s primary healthcare: Findings and implications. Int. J. Qual. Health Care. 2018;31:338–345. doi: 10.1093/intqhc/mzy172. [DOI] [PubMed] [Google Scholar]
- 168.Versporten A., Zarb P., Caniaux I., Gros M.-F., Drapier N., Miller M., Jarlier V., Nathwani D., Goossens H., Koraqi A., et al. Antimicrobial consumption and resistance in adult hospital inpatients in 53 countries: Results of an internet-based global point prevalence survey. Lancet Glob. Health. 2018;6:e619–e629. doi: 10.1016/S2214-109X(18)30186-4. [DOI] [PubMed] [Google Scholar]
- 169.Nakwatumbah S., Kibuule D., Godman B., Haakuria V., Kalemeera F., Baker A., Mubita M., Mwangana M. Compliance to guidelines for the prescribing of antibiotics in acute infections at Namibia’s national referral hospital: A pilot study and the implications. Expert Rev. Anti-Infect. Ther. 2017;15:713–721. doi: 10.1080/14787210.2017.1320220. [DOI] [PubMed] [Google Scholar]
- 170.Niaz Q., Godman B., Campbell S., Kibuule D. Compliance to prescribing guidelines among public health care facilities in Namibia; findings and implications. Int. J. Clin. Pharm. 2020;42:1227–1236. doi: 10.1007/s11096-020-01056-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Afriyie D.K., Sefah I.A., Sneddon J., Malcolm W., McKinney R., Cooper L., Kurdi A., Godman B., Seaton R.A. Antimicrobial point prevalence surveys in two Ghanaian hospitals: Opportunities for antimicrobial stewardship. JAC Antimicrob. Resist. 2020;2:1–9. doi: 10.1093/jacamr/dlaa001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Mashalla Y., Setlhare V., Massele A., Sepako E., Tiroyakgosi C., Kgatlwane J., Chuma M., Godman B. Assessment of prescribing practices at the primary healthcare facilities in Botswana with an emphasis on antibiotics: Findings and implications. Int. J. Clin. Pract. 2017;71:e13042. doi: 10.1111/ijcp.13042. [DOI] [PubMed] [Google Scholar]
- 173.Sharland M., Gandra S., Huttner B., Moja L., Pulcini C., Zeng M., Mendelson M., Cappello B., Cooke G., Magrini N., et al. Encouraging AWaRe-ness and discouraging inappropriate antibiotic use—The new 2019 Essential Medicines List becomes a global antibiotic stewardship tool. Lancet Infect. Dis. 2019;19:1278–1280. doi: 10.1016/S1473-3099(19)30532-8. [DOI] [PubMed] [Google Scholar]
- 174.Saleem Z., Hassali M.A., Godman B., Fatima M., Ahmad Z., Sajid A., Rehman I.U., Nadeem M.U., Javaid Z., Malik M., et al. Sale of WHO AWaRe groups antibiotics without a prescription in Pakistan: A simulated client study. J. Pharm. Policy Pract. 2020;13:26. doi: 10.1186/s40545-020-00233-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Hsia Y., Lee B.R., Versporten A., Yang Y., Bielicki J., Jackson C., Newland J., Goossens H., Magrini N., Sharland M., et al. Use of the WHO Access, Watch, and Reserve classification to define patterns of hospital antibiotic use (AWaRe): An analysis of paediatric survey data from 56 countries. Lancet Glob. Health. 2019;7:e861–e871. doi: 10.1016/S2214-109X(19)30071-3. [DOI] [PubMed] [Google Scholar]
- 176.Hoffmann M. The right drug, but from whose perspective? A framework for analysing the structure and activities of drug and therapeutics committees. Eur. J. Clin. Pharm. 2013;69(Suppl. 1):79–87. doi: 10.1007/s00228-013-1491-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Matlala M., Gous A.G.S., Meyer J.C., Godman B. Formulary Management Activities and Practice Implications among Public Sector Hospital Pharmaceutical and Therapeutics Committees in a South African Province. Front. Pharm. 2020;11 doi: 10.3389/fphar.2020.01267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Matlala M., Gous A.G., Godman B., Meyer J.C. Structure and activities of pharmacy and therapeutics committees among public hospitals in South Africa; findings and implications. Expert Rev. Clin. Pharm. 2017;10:1273–1280. doi: 10.1080/17512433.2017.1364625. [DOI] [PubMed] [Google Scholar]
- 179.Lee M.H., Lee G.A., Lee S.H., Park Y.H. Effectiveness and core components of infection prevention and control programmes in long-term care facilities: A systematic review. J. Hosp. Infect. 2019;102:377–393. doi: 10.1016/j.jhin.2019.02.008. [DOI] [PubMed] [Google Scholar]
- 180.Mbui J.M., Oluka M.N., Guantai E.M., Sinei K.A., Achieng L., Baker A., Jande M., Massele A., Godman B. Prescription patterns and adequacy of blood pressure control among adult hypertensive patients in Kenya; findings and implications. Expert Rev. Clin. Pharm. 2017;10:1263–1271. doi: 10.1080/17512433.2017.1371590. [DOI] [PubMed] [Google Scholar]
- 181.Matsitse T.B., Helberg E., Meyer J.C., Godman B., Massele A., Schellack N. Compliance with the primary health care treatment guidelines and the essential medicines list in the management of sexually transmitted infections in correctional centres in South Africa: Findings and implications. Expert Rev. Anti-Infect. Ther. 2017;15:963–972. doi: 10.1080/14787210.2017.1382354. [DOI] [PubMed] [Google Scholar]
- 182.Brooks J. US Medicare will stop paying for preventable errors. Can. Med. Assoc. J. 2007;177:841–842. doi: 10.1503/cmaj.071347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Alrasheedy A.A., Alsalloum M.A., Almuqbil F.A., Almuzaini M.A., Aba Alkhayl B.S., Albishri A.S., Alharbi F.F., Alharbi S.R., Alodhayb A.K., Alfadl A.A., et al. The impact of law enforcement on dispensing antibiotics without prescription: A multi-methods study from Saudi Arabia. Expert Rev. Anti-Infect. Ther. 2020;18:87–97. doi: 10.1080/14787210.2020.1705156. [DOI] [PubMed] [Google Scholar]
- 184.Godman B., Basu D., Pillay Y., Almeida P.H., Mwita J.C., Rwegerera G.M., Paramadhas B.D.A., Tiroyakgosi C., Patrick O., Niba L.L., et al. Ongoing and planned activities to improve the management of patients with Type 1 diabetes across Africa; implications for the future. Hosp. Pract. 2020;48:51–67. doi: 10.1080/21548331.2020.1745509. [DOI] [PubMed] [Google Scholar]
- 185.Godman B., Basu D., Pillay Y., Mwita J.C., Rwegerera G.M., Anand Paramadhas B.D., Tiroyakgosi C., Okwen P.M., Niba L.L., Nonvignon J., et al. Review of Ongoing Activities and Challenges to Improve the Care of Patients with Type 2 Diabetes across Africa and the Implications for the Future. Front. Pharm. 2020;11:108. doi: 10.3389/fphar.2020.00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.WHO Health Care-Associated Infections. [(accessed on 7 May 2021)];2014 FACT SHEET. Available online: https://www.who.int/gpsc/country_work/gpsc_ccisc_fact_sheet_en.pdf.
- 187.Mugomeri E. The efficacy of infection prevention and control committees in Lesotho: A qualitative study. Am. J. Infect. Control. 2018;46:e13–e17. doi: 10.1016/j.ajic.2017.11.028. [DOI] [PubMed] [Google Scholar]
- 188.Gilbert G.L., Kerridge I. The politics and ethics of hospital infection prevention and control: A qualitative case study of senior clinicians’ perceptions of professional and cultural factors that influence doctors’ attitudes and practices in a large Australian hospital. BMC Health Serv. Res. 2019;19:212. doi: 10.1186/s12913-019-4044-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Mpinda-Joseph P., Anand Paramadhas B.D., Reyes G., Maruatona M.B., Chise M., Monokwane-Thupiso B.B., Souda S., Tiroyakgosi C., Godman B. Healthcare-associated infections including neonatal bloodstream infections in a leading tertiary hospital in Botswana. Hosp. Pract. 2019;47:203–210. doi: 10.1080/21548331.2019.1650608. [DOI] [PubMed] [Google Scholar]
- 190.WHO Minimum Requirements for Infection Prevention and Control Programmes. [(accessed on 8 May 2021)];2019 Available online: https://www.who.int/publications/i/item/9789241516945.
- 191.Li M., Wang X., Wang J., Tan R., Sun J., Li L., Huang J., Wu J., Gu Q., Zhao Y., et al. Infection-prevention and control interventions to reduce colonisation and infection of intensive care unit-acquired carbapenem-resistant Klebsiella pneumoniae: A 4-year quasi-experimental before-and-after study. Antimicrob. Resist. Infect. Control. 2019;8:8. doi: 10.1186/s13756-018-0453-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Infection Control Africa Network (ICAN) Training Course: Infection Prevention and Control for Healthcare Workers 12–16 April 2021. [(accessed on 8 May 2021)]; Available online: http://www.icanetwork.co.za/training-course-infection-prevention-and-control-for-healthcare-workers-12-16-april-2021/
- 193.Kurdi A., Hasan A.J., Baker K.I., Seaton R.A., Ramzi Z.S., Sneddon J., Godman B. A multicentre point prevalence survey of hospital antibiotic prescribing and quality indices in the Kurdistan Regional Government of Northern Iraq: The need for urgent action. Expert Rev. Anti-Infect. Ther. 2021;19:805–814. doi: 10.1080/14787210.2021.1834852. [DOI] [PubMed] [Google Scholar]
- 194.Momanyi L., Opanga S., Nyamu D., Oluka M., Kurdi A., Godman B. Antibiotic prescribing patterns at a leading referral hospital in Kenya: A point prevalence survey. J. Res. Pharm. Pract. 2019;8:149–154. doi: 10.4103/jrpp.jrpp_18_68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Allegranzi B., Bagheri Nejad S., Chraiti M., Combescure C., Attar H., Pittet D. Report on the Burden of Endemic Health Care-Associated Infection Worldwide. World Health Organization; Geneva, Switzerland: 2011. [Google Scholar]
- 196.Prevention E.C.f.D., Control Suetens C., Hopkins S., Kolman J., Högberg L.D. Point Prevalence Survey of Healthcare-Associated Infections and Antimicrobial Use in European Acute Care Hospitals: 2011–2012. Publications Office of the European Union; Luxembourg: 2013. [Google Scholar]
- 197.Nejad S.B., Allegranzi B., Syed S.B., Ellis B., Pittet D. Health-care-associated infection in Africa: A systematic review. Bull. WHO. 2011;89:757–765. doi: 10.2471/BLT.11.088179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Rothe C., Schlaich C., Thompson S. Healthcare-associated infections in sub-Saharan Africa. J. Hosp. Infect. 2013;85:257–267. doi: 10.1016/j.jhin.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 199.Umscheid C.A., Mitchell M.D., Doshi J.A., Agarwal R., Williams K., Brennan P.J. Estimating the Proportion of Healthcare-Associated Infections That Are Reasonably Preventable and the Related Mortality and Costs. Infect. Control Hosp. Epidemiol. 2011;32:101–114. doi: 10.1086/657912. [DOI] [PubMed] [Google Scholar]
- 200.Manoukian S., Stewart S., Dancer S., Graves N., Mason H., McFarland A., Robertson C., Reilly J. Estimating excess length of stay due to healthcare-associated infections: A systematic review and meta-analysis of statistical methodology. J. Hosp. Infect. 2018;100:222–235. doi: 10.1016/j.jhin.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 201.Ataiyero Y., Dyson J., Graham M. Barriers to hand hygiene practices among health care workers in sub-Saharan African countries: A narrative review. Am. J. Infect. Control. 2019;47:565–573. doi: 10.1016/j.ajic.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 202.Afriyie D.K., Amponsah S.K., Dogbey J., Agyekum K., Kesse S., Truter I., Meyer J.C., Godman B. A pilot study evaluating the prescribing of ceftriaxone in hospitals in Ghana: Findings and implications. Hosp. Pract. 2017;45:143–149. doi: 10.1080/21548331.2017.1348139. [DOI] [PubMed] [Google Scholar]
- 203.Dlamini N.N., Meyer J.C., Kruger D., Kurdi A., Godman B., Schellack N. Feasibility of using point prevalence surveys to assess antimicrobial utilisation in public hospitals in South Africa: A pilot study and implications. Hosp. Pract. 2019;47:88–95. doi: 10.1080/21548331.2019.1592880. [DOI] [PubMed] [Google Scholar]
- 204.Kruger D., Dlamini N.N., Meyer J.C., Godman B., Kurdi A., Lennon M., Bennie M., Schellack N. Development of a web-based application to improve data collection of antimicrobial utilization in the public health care system in South Africa. Hosp. Pract. 2021;2021:1–10. doi: 10.1080/21548331.2021.1889213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Skosana P.P., Schellack N., Godman B., Kurdi A., Bennie M., Kruger D., Meyer J. A point prevalence survey of antimicrobial utilisation patterns and quality indices amongst hospitals in South Africa; findings and implications. Expert Rev. Anti-Infect. Ther. 2021;2021:1–13. doi: 10.1080/14787210.2021.1898946. [DOI] [PubMed] [Google Scholar]
- 206.BSAC Practical Guide to Antimicrobial Stewardship in Hospitals. [(accessed on 7 May 2021)];2013 Available online: http://bsac.org.uk/wp-content/uploads/2013/07/Stewardship-Booklet-Practical-Guide-to-Antimicrobial-Stewardship-in-Hospitals.pdf.
- 207.BSAC Antimicrobial Stewardship from Principles to Practice. [(accessed on 7 May 2021)];2018 Available online: http://www.bsac.org.uk/antimicrobialstewardshipebook/BSAC-AntimicrobialStewardship-FromPrinciplestoPractice-eBook.pdf.
- 208.Commonwealth Partnerships for Antimicrobial Stewardship Working Together to Tackle Antimicrobial Resistance in the Commonwealth. [(accessed on 8 May 2021)];2020 Available online: https://commonwealthpharmacy.org/commonwealth-partnerships-for-antimicrobial-stewardship/
- 209.World Health Organization Antimicrobial Stewardship Programmes in Health-Care Facilities in Low- and Mid-Dle-Income Countries. A Who Practical Toolkit. [(accessed on 10 May 2021)];2019 Available online: https://apps.who.int/iris/bitstream/handle/10665/329404/9789241515481-eng.pdf.
- 210.Pulcini C., Binda F., Lamkang A.S., Trett A., Charani E., Goff D., Harbarth S., Hinrichsen S., Levy-Hara G., Mendelson M., et al. Developing core elements and checklist items for global hospital antimicrobial stewardship programmes: A consensus approach. Clin. Microbiol. Infect. 2019;25:20–25. doi: 10.1016/j.cmi.2018.03.033. [DOI] [PubMed] [Google Scholar]
- 211.CDC The Core Elements of Human Antibiotic Stewardship Programs in Resource-Limited Settings: National and Hospital Levels. [(accessed on 8 May 2021)]; Available online: https://www.cdc.gov/antibiotic-use/healthcare/implementation.html.
- 212.Vu T.L., Vu Q.D., Hoang B.L., Nguyen T.C., Ta T.D., Nadjm B., van Doorn H.R. Factors influencing choices of empirical antibiotic treatment for bacterial infections in a scenario-based survey in Vietnam. JAC-Antimicrob. Resist. 2020;2:dlaa087. doi: 10.1093/jacamr/dlaa087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Kakkar A.K., Shafiq N., Singh G., Ray P., Gautam V., Agarwal R., Muralidharan J., Arora P. Antimicrobial Stewardship Programs in Resource Constrained Environments: Understanding and Addressing the Need of the Systems. Front. Public Health. 2020;8:140. doi: 10.3389/fpubh.2020.00140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Fadare J.O., Ogunleye O., Iliyasu G., Adeoti A., Schellack N., Engler D., Massele A., Godman B. Status of antimicrobial stewardship programmes in Nigerian tertiary healthcare facilities: Findings and implications. J. Glob. Antimicrob. Resist. 2019;17:132–136. doi: 10.1016/j.jgar.2018.11.025. [DOI] [PubMed] [Google Scholar]
- 215.Kalungia A.C., Mwambula H., Munkombwe D., Marshall S., Schellack N., May C., Jones A.S.C., Godman B. Antimicrobial stewardship knowledge and perception among physicians and pharmacists at leading tertiary teaching hospitals in Zambia: Implications for future policy and practice. J. Chemother. 2019;31:378–387. doi: 10.1080/1120009X.2019.1622293. [DOI] [PubMed] [Google Scholar]
- 216.Gebretekle G.B., Haile Mariam D.H., Abebe W., Amogne W., Tenna A., Fenta T.G., Libman M., Yansouni C.P., Semret M. Opportunities and barriers to implementing antibiotic stewardship in low and middle-income countries: Lessons from a mixed-methods study in a tertiary care hospital in Ethiopia. PLoS ONE. 2018;13:e0208447. doi: 10.1371/journal.pone.0208447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Schellack N., Bronkhorst E., Coetzee R., Godman B., Gous A.G.S., Kolman SCoetzee R., Bronkhorst E. SASOCP position statement on the pharmacist’s role in antibiotic stewardship South African. J. Infect. Dis. 2018;33:28–35. [Google Scholar]
- 218.Okoth C., Opanga S., Okalebo F., Oluka M., Baker Kurdi A., Godman B. Point prevalence survey of antibiotic use and resistance at a referral hospital in Kenya: Findings and implications. Hosp. Pract. 2018;46:128–136. doi: 10.1080/21548331.2018.1464872. [DOI] [PubMed] [Google Scholar]
- 219.Lew K.Y., Ng T.M., Tan M., Tan S.H., Lew E.L., Ling L.M., Ang B., Lye D., Teng C.B. Safety and clinical outcomes of carbapenem de-escalation as part of an antimicrobial stewardship programme in an ESBL-endemic setting. J. Antimicrob. Chemother. 2014;70:1219–1225. doi: 10.1093/jac/dku479. [DOI] [PubMed] [Google Scholar]
- 220.Stanic Benic M., Milanic R., Monnier A.A., Gyssens I.C., Adriaenssens N., Versporten A., Zanichelli V., Le Maréchal M., Huttner B., Tebano G., et al. Metrics for quantifying antibiotic use in the hospital setting: Results from a systematic review and international multidisciplinary consensus procedure. J. Antimicrob. Chemother. 2018;73(Suppl. 6):vi50–vi58. doi: 10.1093/jac/dky118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Mauger B., Marbella A., Pines E., Chopra R., Black E.R., Aronson N. Implementing quality improvement strategies to reduce healthcare-associated infections: A systematic review. Am. J. Infect. Control. 2014;42:S274–S283. doi: 10.1016/j.ajic.2014.05.031. [DOI] [PubMed] [Google Scholar]
- 222.Luangasanatip N., Hongsuwan M., Limmathurotsakul D., Lubell Y., Lee A.S., Harbarth S., Day N.P., Graves N., Cooper B.S. Comparative efficacy of inter-ventions to promote hand hygiene in hospital: Systematic review and network meta-analysis. BMJ. 2015;351:h3728. doi: 10.1136/bmj.h3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Loftus M.J., Guitart C., Tartari E., Stewardson A.J., Amer F., Bellissimo-Rodrigues F., Lee Y.F., Mehtar S., Sithole B.L., Pittet D. Hand hygiene in low- and middle-income countries. Int. J. Infect. Dis. 2019;86:25–30. doi: 10.1016/j.ijid.2019.06.002. [DOI] [PubMed] [Google Scholar]
- 224.WHO. [(accessed on 8 May 2021)];Hand Hygiene Self-Assessment Framework. Available online: https://www.who.int/gpsc/country_work/hhsa_framework_October_2010.pdf?ua=1.
- 225.Malhotra N.R., Piazza M., Demoor R., McClintock S.D., Hamilton K., Sharma N., Osiemo B., Berger I., Hossain E., Borovskiy Y., et al. Impact of Reduced Preincision Antibiotic Infusion Time on Surgical Site Infection Rates: A Retrospective Cohort Study. Ann. Surg. 2020;271:774–780. doi: 10.1097/SLA.0000000000003030. [DOI] [PubMed] [Google Scholar]
- 226.Najjar P.A., Smink D.S. Prophylactic Antibiotics and Prevention of Surgical Site Infections. Surg. Clin. N. Am. 2015;95:269–283. doi: 10.1016/j.suc.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 227.de Jonge S.W., Gans S.L., Atema J.J., Solomkin J.S., Dellinger P.E., Boermeester M.A. Timing of preoperative antibiotic prophylaxis in 54,552 patients and the risk of surgical site infection: A systematic review and meta-analysis. Medicine. 2017;96:e6903. doi: 10.1097/MD.0000000000006903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Branch-Elliman W., O’Brien W., Strymish J., Itani K., Wyatt C., Gupta K. Association of Duration and Type of Surgical Prophylaxis with Antimicrobial-Associated Adverse Events. JAMA Surg. 2019;154:590–598. doi: 10.1001/jamasurg.2019.0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Saito H., Inoue K., Ditai J., Weeks A.D. Pattern of Peri-Operative Antibiotic Use among Surgical Patients in a Regional Referral and Teaching Hospital in Uganda. Surg. Infect. 2020;21:540–546. doi: 10.1089/sur.2019.176. [DOI] [PubMed] [Google Scholar]
- 230.Harbarth S., Samore M.H., Lichtenberg D., Carmeli Y. Prolonged Antibiotic Prophylaxis after Cardiovascular Surgery and Its Effect on Surgical Site Infections and Antimicrobial Resistance. Circulation. 2000;101:2916–2921. doi: 10.1161/01.CIR.101.25.2916. [DOI] [PubMed] [Google Scholar]
- 231.Hawn M.T., Knowlton L.M. Balancing the Risks and Benefits of Surgical Prophylaxis: Timing and Duration Do Matter. JAMA Surg. 2019;154:598–599. doi: 10.1001/jamasurg.2019.0570. [DOI] [PubMed] [Google Scholar]
- 232.Bull A.L., Worth L.J., Spelman T., Richards M.J. Antibiotic Prescribing Practices for Prevention of Surgical Site Infections in Australia: Increased Uptake of National Guidelines after Surveillance and Reporting and Impact on Infection Rates. Surg. Infect. 2017;18:834–840. doi: 10.1089/sur.2017.119. [DOI] [PubMed] [Google Scholar]
- 233.Langerman A., Thisted R., Hohmann S., Howell M. Antibiotic and Duration of Perioperative Prophylaxis Predicts Surgical Site Infection in Head and Neck Surgery. Otolaryngol. Neck Surg. 2016;154:1054–1063. doi: 10.1177/0194599816634303. [DOI] [PubMed] [Google Scholar]
- 234.Vicentini C., Politano G., Corcione S., Furmenti M.F., Quattrocolo F., De Rosa F.G., Zotti C.M. Surgical antimicrobial prophylaxis prescribing practices and impact on infection risk: Results from a multicenter surveillance study in Italy (2012–2017) Am. J. Infect. Control. 2019;47:1426–1430. doi: 10.1016/j.ajic.2019.07.013. [DOI] [PubMed] [Google Scholar]
- 235.Nthumba P.M. Effective Hand Preparation for Surgical Procedures in Low- and Middle-Income Countries. Surg. Infect. 2020;21:495–500. doi: 10.1089/sur.2020.025. [DOI] [PubMed] [Google Scholar]
- 236.Davey P., Peden C., Brown E., Charani E., Michie S., Ramsay C.R., Marwick C.A. Interventions to improve antibiotic prescribing practices for hospital inpatients (updated protocol) Cochrane Database Syst. Rev. 2014;2:Cd003543. doi: 10.1002/14651858.cd011236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.McLeod M., Ahmad R., Shebl N.A., Micallef C., Sim F., Holmes A. A whole-health–economy approach to antimicrobial stewardship: Analysis of current models and future direction. PLoS Med. 2019;16:e1002774. doi: 10.1371/journal.pmed.1002774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Curtis C.E., Al Bahar F., Marriott J.F. The effectiveness of computerised decision support on antibiotic use in hospitals: A systematic review. PLoS ONE. 2017;12:e0183062. doi: 10.1371/journal.pone.0183062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Tahoon M.A., Khalil M.M., Hammad E., Morad W.S., Awad S.M., Ezzat S. The effect of educational intervention on healthcare providers’ knowledge, attitude, & practice towards antimicrobial stewardship program at, National Liver Institute, Egypt. Egypt. Liver J. 2020;10:5. [Google Scholar]
- 240.Majumder M.A.A., Singh K., Hilaire M.G., Rahman S., Sa B., Haque M. Tackling Antimicrobial Resistance by promoting Anti-microbial stewardship in Medical and Allied Health Professional Curricula. Expert Rev. Anti-Infect. Ther. 2020;18:1245–1258. doi: 10.1080/14787210.2020.1796638. [DOI] [PubMed] [Google Scholar]
- 241.Bozkurt F., Kaya S., Tekin R., Gulsun S., Deveci O., Dayan S., Hosoglu S. Analysis of antimicrobial consumption and cost in a teaching hospital. J. Infect. Public Health. 2014;7:161–169. doi: 10.1016/j.jiph.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 242.Hou D., Wang Q., Jiang C., Tian C., Li H., Ji B. Evaluation of the Short-Term Effects of Antimicrobial Stewardship in the Intensive Care Unit at a Tertiary Hospital in China. PLoS ONE. 2014;9:e101447. doi: 10.1371/journal.pone.0101447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Amdany H.K., McMillan M. Metronidazole intravenous formulation use in in-patients in Kapkatet District Hospital, Kenya: A best practice implementation project. JBI Database Syst. Rev. Implement. Rep. 2014;12:419–432. doi: 10.11124/jbisrir-2014-1381. [DOI] [Google Scholar]
- 244.Yang Z., Zhao P., Wang J., Tong L., Cao J., Tian Y., Yao Z., Wang J., Zhu Y., Jia Y., et al. DRUGS System Enhancing Adherence of Chinese Surgeons to Antibiotic Use Guidelines during Perioperative Period. PLoS ONE. 2014;9:e102226. doi: 10.1371/journal.pone.0102226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Okumura L.M., Da Silva M.M.G., Veroneze I. Effects of a bundled Antimicrobial Stewardship Program on mortality: A cohort study. Braz. J. Infect. Dis. 2015;19:246–252. doi: 10.1016/j.bjid.2015.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Okumura L.M., Riveros B.S., Gomes-da-Silva M.M., Veroneze I. A cost-effectiveness analysis of two different antimicrobial stew-ardship programs. Braz. J. Infect. Dis. Off. Publ. Braz. Soc. Infect. Dis. 2016;20:255–261. doi: 10.1016/j.bjid.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Saied T., Hafez S.F., Kandeel A., El-Kholy A., Ismail G., Aboushady M., Attia E., Hassaan A., Abdel-Atty O., Elfekky E., et al. Antimicrobial stewardship to optimize the use of antimicrobials for surgical prophylaxis in Egypt: A multicenter pilot intervention study. Am. J. Infect. Control. 2015;43:e67–e71. doi: 10.1016/j.ajic.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 248.Ntumba P., Mwangi C., Barasa J., Aiken A.M., Kubilay Z., Allegranzi B. Multimodal approach for surgical site infection prevention—Results from a pilot site in Kenya. Antimicrob. Resist. Infect. Control. 2015;4:87. doi: 10.1186/2047-2994-4-S1-P87. [DOI] [Google Scholar]
- 249.Apisarnthanarak A., Lapcharoen P., Vanichkul P., Srisaeng-Ngoen T., Mundy L.M. Design and analysis of a pharmacist-enhanced antimicrobial stewardship program in Thailand. Am. J. Infect. Control. 2015;43:956–959. doi: 10.1016/j.ajic.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 250.Boyles T.H., Naicker V., Rawoot N., Raubenheimer P.J., Eick B., Mendelson M. Sustained reduction in antibiotic consumption in a South African public sector hospital; Four year outcomes from the Groote Schuur Hospital antibiotic stewardship program. S. Afr. Med. J. 2017;107:115–118. doi: 10.7196/SAMJ.2017.v107i2.12067. [DOI] [PubMed] [Google Scholar]
- 251.Brink A.J., Messina A.P., Feldman C., Richards G.A., van den Bergh D. From guidelines to practice: A pharmacist-driven prospective audit and feedback improvement model for perioperative antibiotic prophylaxis in 34 South African hospitals. J. Antimicrob. Chemother. 2016;72:1227–1234. doi: 10.1093/jac/dkw523. [DOI] [PubMed] [Google Scholar]
- 252.Allegranzi B., Aiken A.M., Zeynep Kubilay N., Nthumba P., Barasa J., Okumu G., Mugarura R., Elobu A., Jombwe J., Maimbo M., et al. A multimodal infection control and patient safety intervention to reduce surgical site infections in Africa: A multicentre, before–after, cohort study. Lancet Infect. Dis. 2018;18:507–515. doi: 10.1016/S1473-3099(18)30107-5. [DOI] [PubMed] [Google Scholar]
- 253.Khdour M.R., Hallak H.O., Aldeyab M.A., Nasif M.A., Khalili A.M., Dallashi A.A., Khofash M.B., Scott M.G. Impact of antimicrobial stewardship programme on hospitalized patients at the intensive care unit: A prospective audit and feedback study. Br. J. Clin. Pharmacol. 2018;84:708–715. doi: 10.1111/bcp.13486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Abubakar U., Syed Sulaiman S.A., Adesiyun A.G. Impact of pharmacist-led antibiotic stewardship interventions on compliance with surgical antibiotic prophylaxis in obstetric and gynecologic surgeries in Nigeria. PLoS ONE. 2019;14:e0213395. doi: 10.1371/journal.pone.0213395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Karaali C., Emiroglu M., Atalay S., Sert I., Dursun A., Kose S., Akbulut G., Aydın C. A new antibiotic stewardship program approach is effective on inappropriate surgical prophylaxis and discharge prescription. J. Infect. Dev. Ctries. 2019;13:961–967. doi: 10.3855/jidc.11734. [DOI] [PubMed] [Google Scholar]
- 256.Mahmoudi L., Ghouchani M., Mahi-Birjand M., Bananzadeh A., Akbari A. Optimizing compliance with surgical antimicrobial prophylaxis guidelines in patients undergoing gastrointestinal surgery at a referral teaching hospital in southern Iran: Clinical and economic impact. Infect. Drug Resist. 2019;12:2437–2444. doi: 10.2147/IDR.S212728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Xiao Y., Shen P., Zheng B., Zhou K., Luo Q., Li L. Change in Antibiotic Use in Secondary and Tertiary Hospitals Nationwide after a National Antimicrobial Stewardship Campaign Was Launched in China, 2011–2016: An Observational Study. J. Infect. Dis. 2020;221:S148–S155. doi: 10.1093/infdis/jiz556. [DOI] [PubMed] [Google Scholar]
- 258.Mardani M., Abolghasemi S., Shabani S. Impact of an antimicrobial stewardship program in the antimicrobial-resistant and prevalence of clostridioides difficile infection and amount of antimicrobial consumed in cancer patients. BMC Res. Notes. 2020;13:246. doi: 10.1186/s13104-020-05085-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.van den Bergh D., Messina A.P., Goff D.A., van Jaarsveld A., Coetzee R., de Wet Y., Bronkhorst E., Brink A., Mendelson M., Richards G.A. A pharmacist-led prospective antibiotic stewardship intervention improves compliance to community-acquired pneumonia guidelines in 39 public and private hospitals across South Africa. Int. J. Antimicrob. Agents. 2020;56:106189. doi: 10.1016/j.ijantimicag.2020.106189. [DOI] [PubMed] [Google Scholar]
- 260.Dinh A., Duran C., Davido B., Bouchand F., Deconinck L., Matt M., Sénard O., Guyot C., Levasseur A.-S., Attal J., et al. Impact of an antimicrobial stewardship programme to optimize antimicrobial use for outpatients at an emergency department. J. Hosp. Infect. 2017;97:288–293. doi: 10.1016/j.jhin.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 261.Bishop B.M. Antimicrobial Stewardship in the Emergency Department: Challenges, Opportunities, and a Call to Action for Pharmacists. J. Pharm. Pract. 2015;29:556–563. doi: 10.1177/0897190015585762. [DOI] [PubMed] [Google Scholar]
- 262.Acosta A., Vanegas E.P., Rovira J., Godman B., Bochenek T. Medicine Shortages: Gaps between Countries and Global Perspectives. Front. Pharm. 2019;10 doi: 10.3389/fphar.2019.00763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Modisakeng C., Matlala M., Godman B., Meyer J.C. Medicine shortages and challenges with the procurement process among public sector hospitals in South Africa; findings and implications. BMC Health Serv. Res. 2020;20:234. doi: 10.1186/s12913-020-05080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Chigome A.K., Matlala M., Godman B., Meyer J.C. Availability and Use of Therapeutic Interchange Policies in Managing Antimicrobial Shortages among South African Public Sector Hospitals; Findings and Implications. Antibiotics. 2019;9:4. doi: 10.3390/antibiotics9010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Gundlapalli A.V., Beekmann S.E., Graham D.R., Polgreen P.M. Perspectives and concerns regarding antimicrobial agent shortages among infectious disease specialists. Diagn. Microbiol. Infect. Dis. 2013;75:256–259. doi: 10.1016/j.diagmicrobio.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Pulcini C., Beovic B., Béraud G., Carlet J., Cars O., Howard P., Levy-Hara G., Li G., Nathwani D., Roblot F., et al. Ensuring universal access to old antibiotics: A critical but neglected priority. Clin. Microbiol. Infect. 2017;23:590–592. doi: 10.1016/j.cmi.2017.04.026. [DOI] [PubMed] [Google Scholar]
- 267.Schellack N., Coetzee M., Schellack G., Gijzelaar M., Hassim Z., Milne M., Bronkhorst E., Padayachee N., Singh N., Kolman S., et al. COVID-19: Guidelines for pharmacists in South Africa. S. Afr. J. Infect. Dis. 2020;35:10. doi: 10.4102/sajid.v35i1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Uzochukwu B., Onwujekwe O., Eriksson B. Inequity in the Bamako Initiative programme—Implications for the treatment of malaria in south-east Nigeria. Int. J. Health Plan. Manag. 2004;19:S107–S116. doi: 10.1002/hpm.779. [DOI] [PubMed] [Google Scholar]
- 269.Omololu F.O., Okunola R.A., Salami K.K. Equity and access to health care services: The experience of the Bamako initiative programme in Nigeria. J. Med. Med. Sci. 2012;3:434–442. [Google Scholar]
- 270.Ogbonna B.O., Nwako N.N. Essential Drugs Revolving Fund Scheme in Nigeria; from the Edge of a Precipice towards Sustainability. J. Adv. Med. Pharm. Sci. 2016;8:1–8. doi: 10.9734/JAMPS/2016/25950. [DOI] [Google Scholar]
- 271.Shaikhan F., Rawaf S., Majeed A., Hassounah S. Knowledge, attitude, perception and practice regarding antimicrobial use in upper respiratory tract infections in Qatar: A systematic review. JRSM Open. 2018;9:2054270418774971. doi: 10.1177/2054270418774971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Erku D.A., Mekuria A.B., Belachew S.A. Inappropriate use of antibiotics among communities of Gondar town, Ethiopia: A threat to the development of antimicrobial resistance. Antimicrob. Resist. Infect. Control. 2017;6:112. doi: 10.1186/s13756-017-0272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Wei X., Zhang Z., Hicks J.P., Walley J.D., King R., Newell J.N., Yin J., Zeng J., Guo Y., Lin M., et al. Long-term outcomes of an educational intervention to reduce antibiotic prescribing for childhood upper respiratory tract infections in rural China: Follow-up of a cluster-randomised controlled trial. PLoS Med. 2019;16:e1002733. doi: 10.1371/journal.pmed.1002733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Holloway K.A., Ivanovska V., Wagner A.K., Vialle-Valentin C., Ross-Degnan D. Prescribing for acute childhood infections in developing and transitional countries, 1990–2009. Paediatr. Int. Child Health. 2015;35:5–13. doi: 10.1179/2046905514Y.0000000115. [DOI] [PubMed] [Google Scholar]
- 275.Kibuule D., Kagoya H.R., Godman B. Antibiotic use in acute respiratory infections in under-fives in Uganda: Findings and implications. Expert Rev. Anti-Infect. Ther. 2016;14:863–872. doi: 10.1080/14787210.2016.1206468. [DOI] [PubMed] [Google Scholar]
- 276.Farley E., Stewart A., Davies M.-A., Govind M., Van den Bergh D., Boyles T.H. Antibiotic use and resistance: Knowledge, attitudes and perceptions among primary care prescribers in South Africa. S. Afr. Med. J. 2018;108:763–771. doi: 10.7196/SAMJ.2018.v108i9.12933. [DOI] [PubMed] [Google Scholar]
- 277.Teng C.L., Achike F.I., Phua K.L., Nurjahan M.I., Mastura I., Asiah H.N., Mariam A.M., Narayanan S., Norsiah A., Sabariah I., et al. Modifying antibiotic prescribing: The effectiveness of academic detailing plus information leaflet in a Malaysian primary care setting. Med. J. Malays. 2006;61:323–331. [PubMed] [Google Scholar]
- 278.Shrestha N., Samir K.C., Baltussen R., Kafle K.K., Bishai D., Niessen L. Practical Approach to Lung Health in Nepal: Better prescribing and reduction of cost. Trop. Med. Int. Health. 2006;11:765–772. doi: 10.1111/j.1365-3156.2006.01599.x. [DOI] [PubMed] [Google Scholar]
- 279.Awad A.I., Eltayeb I.B., Baraka O.Z. Changing antibiotics prescribing practices in health centers of Khartoum State, Sudan. Eur. J. Clin. Pharm. 2006;62:135–142. doi: 10.1007/s00228-005-0089-4. [DOI] [PubMed] [Google Scholar]
- 280.Kafle K.K., Bhuju G.B., Karkee S.B., Prasad R.R., Shrestha N., Shrestha A.D., Das P.L., Chataut P.D., Daud M. An intervention improving prescribing practices and monitoring drugs availability in a district. Nepal Med. Coll. J. 2009;11:217–221. [PubMed] [Google Scholar]
- 281.Yip W., Powell-Jackson T., Chen W., Hu M., Fe E., Hu M., Jian W., Lu M., Han W., Hsiao W.C. Capitation combined with pay-for-performance improves antibiotic prescribing practices in rural China. Health Aff. 2014;33:502–510. doi: 10.1377/hlthaff.2013.0702. [DOI] [PubMed] [Google Scholar]
- 282.Boonyasiri A., Thamlikitkul V. Effectiveness of multifaceted interventions on rational use of antibiotics for patients with upper respiratory tract infections and acute diarrhea. J. Med. Assoc. Thail. Chotmaihet Thangphaet. 2014;97(Suppl. 3):S13–S19. [PubMed] [Google Scholar]
- 283.Tay K.H., Ariffin F., Sim B.L., Chin S.Y., Sobry A.C. Multi-Faceted Intervention to Improve the Antibiotic Prescriptions among Doctors for Acute URI and Acute Diarrhoea Cases: The Green Zone Antibiotic Project. Malays. J. Med. Sci. 2019;26:101–109. doi: 10.21315/mjms2019.26.4.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Ofori-Asenso R., Brhlikova P., Pollock A.M. Prescribing indicators at primary health care centers within the WHO African region: A systematic analysis (1995–2015) BMC Public Health. 2016;16:724. doi: 10.1186/s12889-016-3428-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Rwegerera G.M., Shailemo D.H.P., Pina Rivera Y., Mokgosi K.O., Bale P., Oyewo T.A., Luis B.D., Habte D., Godman B. Metabolic Control and Determinants among HIV-Infected Type 2 Diabetes Mellitus Patients Attending a Tertiary Clinic in Botswana. Diabetes Metab. Syndr. Obes. 2021;14:85–97. doi: 10.2147/DMSO.S285720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Mwita J.C., Godman B., Esterhuizen T.M. Statin prescription among patients with type 2 diabetes in Botswana: Findings and implications. BMC Endocr. Disord. 2020;20:36. doi: 10.1186/s12902-020-0516-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Achwoka D., Waruru A., Chen T.-H., Masamaro K., Ngugi E., Kimani M., Mukui I., Oyugi J.O., Mutave R., Achia T., et al. Noncommunicable disease burden among HIV patients in care: A national retrospective longitudinal analysis of HIV-treatment outcomes in Kenya, 2003–2013. BMC Public Health. 2019;19:372. doi: 10.1186/s12889-019-6716-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Appiah L.T., Sarfo F.S., Huffman M.D., Nguah S.B., Stiles J.K. Cardiovascular risk factors among Ghanaian patients with HIV: A cross-sectional study. Clin. Cardiol. 2019;42:1195–1201. doi: 10.1002/clc.23273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Chang A.Y., Gómez-Olivé F.X., Manne-Goehler J., Wade A.N., Tollman S., Gaziano T.A., Salomon J.A. Multimorbidity and care for hypertension, diabetes and HIV among older adults in rural South Africa. Bull. World Health Organ. 2018;97:10–23. doi: 10.2471/BLT.18.217000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Fernandez Urrusuno R., Flores Dorado M., Vilches Arenas A., Serrano Martino C., Corral Baena S., Montero Balosa M.C. Improving the appropriateness of antimicrobial use in primary care after implementation of a local antimicrobial guide in both levels of care. Eur. J. Clin. Pharmacol. 2014;70:1011–1020. doi: 10.1007/s00228-014-1704-z. [DOI] [PubMed] [Google Scholar]
- 291.Kibuule D., Mubita M., Naikaku E., Kalemeera F., Godman B.B., Sagwa E. An analysis of policies for cotrimoxazole, amoxicillin and azithromycin use in Namibia’s public sector: Findings and therapeutic implications. Int. J. Clin. Pract. 2017;71:e12918. doi: 10.1111/ijcp.12918. [DOI] [PubMed] [Google Scholar]
- 292.Mashalla Y.J., Sepako E., Setlhare V., Chuma M., Bulang M., Massele A.Y. Availability of guidelines and policy documents for enhancing performance of practitioners at the Primary Health Care (PHC) facilities in Gaborone, Tlokweng and Mogoditshane, Republic of Botswana. J. Public Health Epidemiol. 2016;8:127–135. [Google Scholar]
- 293.Meyer J.C., Schellack N., Stokes J., Lancaster R., Zeeman H., Defty D., Godman B., Steel G. Ongoing Initiatives to Improve the Quality and Efficiency of Medicine Use within the Public Healthcare System in South Africa; A Preliminary Study. Front. Pharmacol. 2017;8:751. doi: 10.3389/fphar.2017.00751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Rossato L., Negrão F.J., Simionatto S. Could the COVID-19 pandemic aggravate antimicrobial resistance? Am. J. Infect. Control. 2020;48:1129–1130. doi: 10.1016/j.ajic.2020.06.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Ray K.N., Shi Z., Gidengil C.A., Poon S.J., Uscher-Pines L., Mehrotra A. Antibiotic Prescribing During Pediatric Direct-to-Consumer Telemedicine Visits. Pediatrics. 2019;143:e20182491. doi: 10.1542/peds.2018-2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Hayat K., Li P., Rosenthal M., Xu S., Chang J., Gillani A.H., Khan F.U., Sarwar M.R., Ji S., Shi L., et al. Perspective of community pharmacists about community-based antimicrobial stewardship programs. A multicenter cross-sectional study from China. Expert Rev. Anti-Infect. Ther. 2019;17:1043–1050. doi: 10.1080/14787210.2019.1692655. [DOI] [PubMed] [Google Scholar]
- 297.Poyongo B.P., Sangeda R.Z. Pharmacists’ Knowledge, Attitude and Practice Regarding the Dispensing of Antibiotics without Prescription in Tanzania: An Explorative Cross-Sectional Study. Pharmacy. 2020;8:238. doi: 10.3390/pharmacy8040238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Zawahir S., Lekamwasam S., Aslani P. A cross-sectional national survey of community pharmacy staff: Knowledge and anti-biotic provision. PLoS ONE. 2019;14:e0215484. doi: 10.1371/journal.pone.0215484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Haque M., Kumar S., Charan J., Bhatt R., Islam S., Dutta S., Abhayanand J.P., Sharma Y., Sefah I.A., Kurdi A., et al. Utilisation, availability and price changes of medicines and protection equipment for COVID-19 in India: Findings and implications Short title: COVID-19 and price changes of treatments in India. Front. Pharmacol. 2021;11:1822. doi: 10.3389/fphar.2020.582154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Selvaraj S., Farooqui H.H., Karan A. Quantifying the financial burden of households’ out-of-pocket payments on medicines in India: A repeated cross-sectional analysis of National Sample Survey data, 1994–2014. BMJ Open. 2018;8:e018020. doi: 10.1136/bmjopen-2017-018020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Essack S., Bell J., Shephard A. Community pharmacists-Leaders for antibiotic stewardship in respiratory tract infection. J. Clin. Pharm. Ther. 2018;43:302–307. doi: 10.1111/jcpt.12650. [DOI] [PubMed] [Google Scholar]
- 302.Wirtz V., Herrera-Patino J.J., Santa-Ana-Tellez Y., Dreser A., Elseviers M., Vander Stichele R. Analysing policy interventions to prohibit over-the-counter antibiotic sales in four Latin American countries. Trop. Med. Int. Health. 2013;18:665–673. doi: 10.1111/tmi.12096. [DOI] [PubMed] [Google Scholar]
- 303.Vacca C.P., Niño C.Y., Reveiz L. Restriction of antibiotic sales in pharmacies in Bogotá, Colombia: A descriptive study. Rev. Panam. De Salud Pública. 2011;30:586–591. [PubMed] [Google Scholar]
- 304.Godman B., Sakshaug S., Berg C., Wettermark B., Haycox A. Combination of prescribing restrictions and policies to engineer low prices to reduce reimbursement costs. Expert Rev. Pharm. Outcomes Res. 2011;11:121–129. doi: 10.1586/erp.10.87. [DOI] [PubMed] [Google Scholar]
- 305.Voncina L., Strizrep T., Godman B., Bennie M., Bishop I., Campbell S., Vlahović-Palčevski V., Gustafsson L.L. Influence of demand-side measures to enhance ren-in-angiotensin prescribing efficiency in Europe: Implications for the future. Expert Rev. Pharm. Outcomes Res. 2011;11:469–479. doi: 10.1586/erp.11.42. [DOI] [PubMed] [Google Scholar]
- 306.Godman B., Bishop I., Finlayson A.E., Campbell S., Kwon H.-Y., Bennie M. Reforms and initiatives in Scotland in recent years to encourage the prescribing of generic drugs, their influence and implications for other countries. Expert Rev. Pharm. Outcomes Res. 2013;13:469–482. doi: 10.1586/14737167.2013.820956. [DOI] [PubMed] [Google Scholar]
- 307.Santa-Ana-Tellez Y., Mantel-Teeuwisse A.K., Dreser A., Leufkens H.G., Wirtz V.J. Impact of over-the-counter restrictions on antibiotic consumption in Brazil and Mexico. PLoS ONE. 2013;8:e75550. doi: 10.1371/journal.pone.0075550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Moura M.L., Boszczowski I., Mortari N., Barrozo L.V., Chiaravalloti Neto F., Lobo R.D., de Lima A.C., Levin A.S. The Impact of Restricting Over-the-Counter Sales of Antimicrobial Drugs: Preliminary Analysis of National Data. Medicine. 2015;94:e1605. doi: 10.1097/MD.0000000000001605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Lopes-Júnior R., de Sa Del Fiol F., Araujo J.L.O., De Toledo M.I., Barberato-Filho S. Decrease in Penicillin Sales in Brazil after Over-the-Counter Restrictions. Antimicrob. Agents Chemother. 2015;59:5862–5863. doi: 10.1128/AAC.01030-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Mattos K.P.H., Visacri M.B., Quintanilha J.C.F., Lloret G.R., Cursino M.A., Levin A.S., Levy C.E., Moriel P. Brazil’s resolutions to regulate the sale of antibiotics: Impact on consumption and Escherichia coli resistance rates. J. Glob. Antimicrob. Resist. 2017;10:195–199. doi: 10.1016/j.jgar.2017.05.023. [DOI] [PubMed] [Google Scholar]
- 311.Arparsrithongsagul S., Kulsomboon V., Zuckerman I.H. Multidisciplinary Perspective Intervention with Community Involvement to Decrease Antibiotic Sales in Village Groceries in Thailand. Asia Pac. J. Public Health. 2013;27:NP2480–NP2488. doi: 10.1177/1010539513479968. [DOI] [PubMed] [Google Scholar]
- 312.Markovic-Pekovic V., Grubisa N. Self-medication with antibiotics in the Republic of Srpska community pharmacies: Pharmacy staff behavior. Pharmacoepidemiol. Drug Saf. 2012;21:1130–1133. doi: 10.1002/pds.3218. [DOI] [PubMed] [Google Scholar]
- 313.Opanga S.A., Rizvi N., Wamaitha A., Sefah I.A., Godman B. Availability of Medicines in Community Pharmacy to Manage Patients with COVID-19 in Kenya; Pilot Study and Implications. Sch. Acad. J. Pharm. 2021;10:36–42. [Google Scholar]
- 314.Roque F., Soares S., Breitenfeld L., López-Durán A., Figueiras A., Herdeiro M.T. Attitudes of community pharmacists to antibiotic dispensing and microbial resistance: A qualitative study in Portugal. Int. J. Clin. Pharm. 2013;35:417–424. doi: 10.1007/s11096-013-9753-4. [DOI] [PubMed] [Google Scholar]
- 315.Barker A.K., Brown K., Ahsan M., Sengupta S., Safdar N. What drives inappropriate antibiotic dispensing? A mixed-methods study of pharmacy employee perspectives in Haryana, India. BMJ Open. 2017;7:e013190. doi: 10.1136/bmjopen-2016-013190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Hassali M.A., Kamil T.K.T., Md Yusof F.A., Alrasheedy A.A., Yusoff Z.M., Saleem F., Al-Tamimi S.K., Wong Z.Y., Aljadhey H., Godman B. General practitioners’ knowledge, attitude and prescribing of antibiotics for upper respiratory tract infections in Selangor, Malaysia: Findings and implications. Expert Rev. Anti-Infect. Ther. 2015;13:511–520. doi: 10.1586/14787210.2015.1012497. [DOI] [PubMed] [Google Scholar]
- 317.McNulty C.A.M., Nichols T., French D.P., Joshi P., Butler C.C. Expectations for consultations and antibiotics for respiratory tract infection in primary care: The RTI clinical iceberg. Br. J. Gen. Pract. 2013;63:e429–e436. doi: 10.3399/bjgp13X669149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Cross E.L., Tolfree R., Kipping R. Systematic review of public-targeted communication interventions to improve antibiotic use. J. Antimicrob. Chemother. 2016;72:975–987. doi: 10.1093/jac/dkw520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Lim K.K., Teh C.C. A Cross Sectional Study of Public Knowledge and Attitude towards Antibiotics in Putrajaya, Malaysia. South. Med. Rev. 2012;5:26–33. [PMC free article] [PubMed] [Google Scholar]
- 320.McCullough A.R., Parekh S., Rathbone J., Del Mar C.B., Hoffmann T.C. A systematic review of the public’s knowledge and beliefs about antibiotic resistance. J. Antimicrob. Chemother. 2016;71:27–33. doi: 10.1093/jac/dkv310. [DOI] [PubMed] [Google Scholar]
- 321.Rather I.A., Kim B.-C., Bajpai V.K., Park Y.-H. Self-medication and antibiotic resistance: Crisis, current challenges, and prevention. Saudi J. Biol. Sci. 2017;24:808–812. doi: 10.1016/j.sjbs.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Monnet D.L., Safrany N., Heine N., Price C. Comment on: A systematic review of the public’s knowledge and beliefs about antibiotic resistance. J. Antimicrob. Chemother. 2016;71:2364–2365. doi: 10.1093/jac/dkw141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Barker A.K., Brown K., Ahsan M., Sengupta S., Safdar N. Social determinants of antibiotic misuse: A qualitative study of community members in Haryana, India. BMC Public Health. 2017;17:333. doi: 10.1186/s12889-017-4261-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Elong Ekambi G.-A., Okalla Ebongue C., Penda I.C., Nnanga Nga E., Mpondo Mpondo E., Eboumbou Moukoko C.E. Knowledge, practices and attitudes on antibiotics use in Cameroon: Self-medication and prescription survey among children, adolescents and adults in private pharmacies. PLoS ONE. 2019;14:e0212875. doi: 10.1371/journal.pone.0212875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325.Ashiru-Oredope D., Hopkins S. Antimicrobial resistance: Moving from professional engagement to public action. J. Antimicrob. Chemother. 2015;70:2927–2930. doi: 10.1093/jac/dkv297. [DOI] [PubMed] [Google Scholar]
- 326.Shallcross L.J., Howard S.J., Fowler T., Davies S.C. Tackling the threat of antimicrobial resistance: From policy to sustainable action. Philos. Trans. R. Soc. B Biol. Sci. 2015;370:20140082. doi: 10.1098/rstb.2014.0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Behaviour Change for Antibiotic Prescribing in Healthcare Settings Literarure Review and Behavioural Analysis. [(accessed on 10 May 2021)]; Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/405031/Behaviour_Change_for_Antibiotic_Prescribing_-_FINAL.pdf.
- 328.WHO Collaborating Centre on Patient Safety The University of Geneva Hospitals and Faculty of Medicine. Evaluation of Antibiotic Awareness Campaigns. [(accessed on 10 May 2021)]; Available online: https://www.who.int/selection_medicines/committees/expert/21/applications/s6_antibiotic_awareness_campaigns.pdf.
- 329.Ivanovska V., Angelovska B., Van Dijk L., Zdravkovska M., Leufkens H.G., Mantel-Teeuwisse A.K. Change in parental knowledge, attitudes and practice of antibiotic use after a national intervention programme. Eur. J. Public Health. 2018;28:724–729. doi: 10.1093/eurpub/ckx240. [DOI] [PubMed] [Google Scholar]
- 330.Huttner B., Goossens H., Verheij T., Harbarth S. Characteristics and outcomes of public campaigns aimed at improving the use of antibiotics in outpatients in high-income countries. Lancet Infect. Dis. 2010;10:17–31. doi: 10.1016/S1473-3099(09)70305-6. [DOI] [PubMed] [Google Scholar]
- 331.LOcal Campaign on Antibiotics ALliance (LOCAAL) study group Doctors and local media: A synergy for public health information? A controlled trial to evaluate the effects of a multifaceted campaign on antibiotic prescribing (protocol) BMC Public Health. 2011;11:816. doi: 10.1186/1471-2458-11-816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Hornik R., Kelly B. Communication and diet: An overview of experience and principles. J. Nutr. Educ. Behav. 2007;39(Suppl. 2):S5–S12. doi: 10.1016/j.jneb.2006.08.020. [DOI] [PubMed] [Google Scholar]
- 333.Ali A.O.A., Prins M.H. Disease and treatment-related factors associated with tuberculosis treatment default in Khartoum State, Sudan: A case-control study. East. Mediterr. Health J. 2017;23:408–414. doi: 10.26719/2017.23.6.408. [DOI] [PubMed] [Google Scholar]
- 334.Ambaw A.D., Alemie G.A., W/Yohannes S.M., Mengesha Z.B. Adherence to antihypertensive treatment and associated factors among patients on follow up at University of Gondar Hospital, Northwest Ethiopia. BMC Public Health. 2012;12:282. doi: 10.1186/1471-2458-12-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Mehra M., Cossrow N., Kambili C., Underwood R., Makkar R., Potluri R. Assessment of tuberculosis burden in China using a dynamic disease simulation model. Int. J. Tuberc. Lung Dis. Off. J. Int. Union Tuberc. Lung Dis. 2013;17:1186–1194. doi: 10.5588/ijtld.12.0959. [DOI] [PubMed] [Google Scholar]
- 336.National Department of Health Republic of South Africa Introduction of New Drugs and Drug Regimens for the Management of Drug Resistant Tuberculosis in South Africa: Policy Framework. [(accessed on 8 May 2021)]; Available online: https://www.nicd.ac.za/assets/files/Introduction%20of%20new%20drugs%20and%20drug%20regimens%20for%20the%20management%20of%20drug%20resistant%20TB%20in%20SA%20-%202015.pdf.
- 337.Edwards A., Oh H., Mircheva I., Hollander C., Joubert K., Schellack N. An Ototoxicity Grading System Within a Mobile App (OtoCalc) for a Resource-Limited Setting to Guide Grading and Management of Drug-Induced Hearing Loss in Patients with Drug-Resistant Tuberculosis: Prospective, Cross-Sectional Case Series. JMIR Mhealth Uhealth. 2020;8:e14036. doi: 10.2196/14036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Knight S.E., Anyachebelu E.J., Geddes R., Maharaj R. Impact of delayed introduction of sulfadoxine-pyrimethamine and arthemeter-lumefantrine on malaria epidemiology in KwaZulu-Natal, South Africa. Trop. Med. Int. Health. 2009;14:1086–1092. doi: 10.1111/j.1365-3156.2009.02333.x. [DOI] [PubMed] [Google Scholar]
- 339.Phillips A.N., Stover J., Cambiano V., Nakagawa F., Jordan M.R., Pillay D., Doherty M., Revill P., Bertagnolio S. Impact of HIV Drug Resistance on HIV/AIDS-Associated Mortality, New Infections, and Antiretroviral Therapy Program Costs in Sub–Saharan Africa. J. Infect. Dis. 2017;215:1362–1365. doi: 10.1093/infdis/jix089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Banek K., Webb E.L., Smith S.J., Chandramohan D., Staedke S.G. Adherence to treatment with artemether—Lumefantrine or amodiaquine—Artesunate for uncomplicated malaria in children in Sierra Leone: A randomized trial. Malar. J. 2018;17:222. doi: 10.1186/s12936-018-2370-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Alipanah N., Jarlsberg L., Miller C., Linh N.N., Falzon D., Jaramillo E., Nahid P. Adherence interventions and outcomes of tuberculosis treatment: A systematic review and meta-analysis of trials and observational studies. PLoS Med. 2018;15:e1002595. doi: 10.1371/journal.pmed.1002595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Becker N., Cordeiro L.S., Poudel K.C., Sibiya T.E., Sayer A.G., Sibeko L.N. Individual, household, and community level barriers to ART adherence among women in rural Eswatini. PLoS ONE. 2020;15:e0231952. doi: 10.1371/journal.pone.0231952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.Marcolino M.S., Oliveira J.A.Q., D’Agostino M., Ribeiro A.L., Alkmim M.B.M., Novillo-Ortiz D. The Impact of mHealth Interventions: Systematic Review of Systematic Reviews. JMIR Mhealth Uhealth. 2018;6:e23. doi: 10.2196/mhealth.8873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Stephens F., Gandhi N.R., Brust J.C.M., Mlisana K., Moodley P., Allana S., Campbell A., Shah S. Treatment Adherence among Persons Receiving Concurrent Multidrug-Resistant Tuberculosis and HIV Treatment in KwaZulu-Natal, South Africa. J. Acquir. Immune Defic. Syndr. 2019;82:124–130. doi: 10.1097/QAI.0000000000002120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Mekonnen H.S., Azagew A.W. Non-adherence to anti-tuberculosis treatment, reasons and associated factors among TB patients attending at Gondar town health centers, Northwest Ethiopia. BMC Res. Notes. 2018;11:691. doi: 10.1186/s13104-018-3789-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Adeoti A.O., Dada M., Elebiyo T., Fadare J., Ojo O. Survey of antiretroviral therapy adherence and predictors of poor adherence among HIV patients in a tertiary institution in Nigeria. Pan Afr. Med. J. 2019;33:277. doi: 10.11604/pamj.2019.33.277.18711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Moomba K., Van Wyk B. Social and economic barriers to adherence among patients at Livingstone General Hospital in Zambia. Afr. J. Prim. Health Care Fam. Med. 2019;11:e1–e6. doi: 10.4102/phcfm.v11i1.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Vagiri R.V., Meyer J.C., Godman B., Gous A.G.S. Relationship between adherence and health-related quality of life among HIV-patients in South Africa: Findings and implications. J. AIDS HIV Res. 2018;10:121–132. [Google Scholar]
- 349.Baxter C., Karim A.S.S. Recent Advances and the Prospect of an HIV Cure. [(accessed on 8 May 2021)]; Available online: https://www.nrf.ac.za/content/recent-advances-and-prospect-hiv-cure.
- 350.Reader J., van der Watt M.E., Taylor D., Le Manach C., Mittal N., Ottilie S., Theron A., Moyo P., Erlank E., Nardini L., et al. Multistage and transmission-blocking targeted antimalarials discovered from the open-source MMV Pandemic Response Box. Nat. Commun. 2021;12:269. doi: 10.1038/s41467-020-20629-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Adam H.J., Richardson S.E., Jamieson F.B., Rawte P., Low D.E., Fisman D.N. Changing epidemiology of invasive Haemophilus influenzae in Ontario, Canada: Evidence for herd effects and strain replacement due to Hib vaccination. Vaccine. 2010;28:4073–4078. doi: 10.1016/j.vaccine.2010.03.075. [DOI] [PubMed] [Google Scholar]
- 352.Heilmann K.P., Rice C.L., Miller A.L., Miller N.J., Beekmann S.E., Pfaller M.A., Richter S.S., Doern G.V. Decreasing prevalence of beta-lactamase production among respiratory tract isolates of Haemophilus influenzae in the United States. Antimicrob. Agents Chemother. 2005;49:2561–2564. doi: 10.1128/AAC.49.6.2561-2564.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Cohen R., Cohen J.F., Chalumeau M., Levy C. Impact of pneumococcal conjugate vaccines for children in high- and non-high-income countries. Expert Rev. Vaccines. 2017;16:625–640. doi: 10.1080/14760584.2017.1320221. [DOI] [PubMed] [Google Scholar]
- 354.Tomczyk S.M., Lynfield R., Schaffner W., Reingold A., Miller L., Petit S., Holtzman C., Zansky S.M., Thomas A., Baumbach J., et al. Prevention of Antibiotic-Nonsusceptible Invasive Pneumococcal Disease With the 13-Valent Pneumococcal Conjugate Vaccine. Clin. Infect. Dis. 2016;62:1119–1125. doi: 10.1093/cid/ciw067. [DOI] [PubMed] [Google Scholar]
- 355.Jokinen J., Rinta-Kokko H., Siira L., Palmu A.A., Virtanen M.J., Nohynek H., Virolainen-Julkunen A., Toropainen M., Nuorti J.P. Impact of Ten-Valent Pneumococcal Conjugate Vaccination on Invasive Pneumococcal Disease in Finnish Children—A Population-Based Study. PLoS ONE. 2015;10:e0120290. doi: 10.1371/journal.pone.0120290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Lewnard J.A., Lo N.C., Arinaminpathy N., Frost I., Laxminarayan R. Childhood vaccines and antibiotic use in low- and middle-income countries. Nature. 2020;581:94–99. doi: 10.1038/s41586-020-2238-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Czech M., Balcerzak M., Antczak A., Byliniak M., Piotrowska-Rutkowska E., Drozd M., Juszczyk G., Religioni U., Vaillancourt R., Merks P. Flu Vaccinations in Pharmacies—A Review of Pharmacists Fighting Pandemics and Infectious Diseases. Int. J. Environ. Res. Public Health. 2020;17:7945. doi: 10.3390/ijerph17217945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.WHO Leveraging Vaccines to Reduce Antibiotic Use and Prevent Antimicrobial Resistance: An Action Framework—Annex to Immunization Agenda 2030. [(accessed on 10 May 2021)];2020 Available online: https://www.who.int/immunization/VACAMR_Action_Framework.pdf?ua=1.
- 359.Vekemans J., Hasso-Agopsowicz M., Kang G., Hausdorff W.P., Fiore A., Tayler E., Klemm E.J., Laxminarayan R., Srikantiah P., Friede M., et al. Leveraging Vaccines to Reduce Antibiotic Use and Prevent Antimicrobial Resistance: A World Health Organization Action Framework. Clin. Infect. Dis. 2021 doi: 10.1093/cid/ciab062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360.Freeman D., Loe B.S., Chadwick A., Vaccari C., Waite F., Rosebrock L., Jenner L., Petit A., Lewandowsky S., Vanderslott S., et al. COVID-19 vaccine hesitancy in the UK: The Oxford coronavirus explanations, attitudes, and narratives survey (Oceans) II. Psychol. Med. 2020:1–15. doi: 10.1017/s0033291720005188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Lewandowsky S., Cook J., Schmid P., Holford D.L., Finn A., Leask J., Thomson A., Lombardi D., Al-Rawi A.K., Amazeen M.A., et al. The COVID-19 Vaccine Communication Handbook. A Practical Guide for Improving Vaccine Communication and Fighting Misinformation. [(accessed on 8 May 2021)];2021 Available online: https://www.richmond.gov.uk/media/20645/the_covid_19_vaccine_communication_handbook.pdf.
- 362.Wood T. Twitter to Start Banning Accounts That Spread Covid-19 Vaccine Misinformation. [(accessed on 7 March 2021)]; Available online: https://www.ladbible.com/news/news-twitter-to-ban-people-who-spread-covid-19-vaccine-misinformation-20210307.
- 363.WHO Countering Misinformation about COVID-19. [(accessed on 11 May 2021)];2020 Available online: https://www.who.int/news-room/feature-stories/detail/countering-misinformation-about-covid-19.
- 364.UK Government Community Champions to Give COVID-19 Vaccine Advice and Boost Take Up. [(accessed on 11 May 2021)];2021 Available online: https://www.gov.uk/government/news/community-champions-to-give-covid-19-vaccine-advice-and-boost-take-up.
- 365.Igoe M. UN Harassment, COVID-19 Misinformation, and Vaccine Efficacy: This Week in Development. [(accessed on 11 March 2021)]; Available online: https://www.devex.com/news/un-harassment-covid-19-misinformation-and-vaccine-efficacy-this-week-in-development-99134.
- 366.WHO . Geneva: 2016. [(accessed on 5 May 2021)]. Patient Engagement: Technical Series on Safer Primary Care. Available online: https://apps.who.int/iris/bitstream/handle/10665/252269/9789241511629-eng.pdf. [Google Scholar]
- 367.The British Psychological Society Delivering Effective Public Health Campaigns during Covid-19. [(accessed on 4 May 2021)];2020 Available online: https://www.bps.org.uk/sites/www.bps.org.uk/files/Policy/Policy%20-%20Files/Delivering%20effective%20public%20health%20campaigns%20during%20Covid-19.pdf.
- 368.Charan J., Kaur R.J., Bhardwaj P., Haque M., Sharma P., Misra S., Godman B. Rapid review of suspected adverse drug events due to remdesivir in the WHO database; findings and implications. Expert Rev. Clin. Pharm. 2021;14:95–103. doi: 10.1080/17512433.2021.1856655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Dyer O. Covid-19: Remdesivir has little or no impact on survival, WHO trial shows. BMJ. 2020;371:m4057. doi: 10.1136/bmj.m4057. [DOI] [PubMed] [Google Scholar]
- 370.WHO WHO Discontinues Hydroxychloroquine and Lopinavir/Ritonavir Treatment Arms for COVID-19. [(accessed on 4 May 2021)];2020 Available online: https://www.who.int/news-room/detail/04-07-2020-who-discontinues-hydroxychloroquine-and-lopinavir-ritonavir-treatment-arms-for-covid-19.
- 371.Horby P., Mafham M., Linsell L., Bell J.L., Staplin N., Emberson J.R., Wiselka M., Ustianowski A., Elmahi E., Prudon B., et al. Effect of Hydroxychloroquine in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2020;383:2030–2040. doi: 10.1056/NEJMoa2022926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., Ruan L., Song B., Cai Y., Wei M., et al. A Trial of Lopinavir—Ritonavir in Adults Hospitalized with Severe Covid-19. N. Engl. J. Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Recovery Trial Statement from the Chief Investigators of the Randomised Evaluation of COVid-19 thERapY (RECOVERY) Trial on Lopinavir-Ritonavir, 29 June 2020. No Clinical Benefit from Use of Lopinavir-Ritonavir in Hospitalised COVID-19 Patients Studied in RECOVERY. [(accessed on 10 May 2021)]; Available online: https://www.recoverytrial.net/files/lopinavir-ritonavir-recovery-statement-29062020_final.pdf.
- 374.Council for International Organizations of Medical Sciences Medicines Assessment during Public Health Emergencies Needs Good Science, Best Practices and Proper Communication. [(accessed on 8 May 2021)];2020 Available online: https://cioms.ch/wp-content/uploads/2020/06/CIOMS_WGXII_Statement.pdf.
- 375.Zahlanie Y., Mang N.S., Lin K., Hynan L.S., Prokesch B.C. Improved Antibiotic Prescribing Practices for Respiratory Infections Through Use of Computerized Order Sets and Educational Sessions in Pediatric Clinics. Open Forum Infect. Dis. 2021;8:ofaa601. doi: 10.1093/ofid/ofaa601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Holstiege J., Mathes T., Pieper D. Effects of computer-aided clinical decision support systems in improving antibiotic prescribing by primary care providers: A systematic review. J. Am. Med. Inform. Assoc. 2015;22:236–242. doi: 10.1136/amiajnl-2014-002886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Riaz H., Godman B.; Hussain, S.; Malik, F.; Mahmood, S.; Shami, A.; Bashir, S. Prescribing of bisphosphonates and antibiotics in Pakistan: Challenges and opportunities for the future. JPHSR. 2015;6:111–121. [Google Scholar]
- 378.Fadare J.O., Oshikoya K.A., Ogunleye O.O., Desalu O.O., Ferrario A., Enwere O.O., Adeoti A., Sunmonu T.A., Massele A., Baker A., et al. Determinants of antibiotic prescribing among doctors in a Nigerian urban tertiary hospital. Hosp. Pract. 2018;47:53–58. doi: 10.1080/21548331.2018.1475997. [DOI] [PubMed] [Google Scholar]
- 379.Fadare J.O., Oshikoya K.A., Ogunleye O.O., Desalu O.O., Ferrario A., Enwere O.O., Adeoti A., Sunmonu T.A., Massele A., Baker A., et al. Drug promotional activities in Nigeria: Impact on the prescribing patterns and practices of medical practitioners and the implications. Hosp. Pract. 2018;46:77–87. doi: 10.1080/21548331.2018.1437319. [DOI] [PubMed] [Google Scholar]
- 380.Adepoju I.-O.O., Albersen B.J.A., De Brouwere V., Van Roosmalen J., Zweekhorst M. mHealth for Clinical Decision-Making in Sub-Saharan Africa: A Scoping Review. JMIR Mhealth Uhealth. 2017;5:e38. doi: 10.2196/mhealth.7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 381.Birjovanu G., Lefevre C., Hayward A., Molnar A., Ncube F., Wiseman S., Kostkova P., Wood C., Olufemi O., Ogunsola F., et al. GADSA: Decision Support App for Antibiotics Prescribing in Nigeria; Proceedings of the 9th International Conference on Digital Public Health; New York, NY, USA. 20–23 November 2019; pp. 9–10. [Google Scholar]
- 382.Charani E., Smith I., Skodvin B., Perozziello A., Lucet J.-C., Lescure F.-X., Birgand G., Poda A., Ahmad R., Singh S., et al. Investigating the cultural and contextual determinants of antimicrobial stewardship programmes across low-, middle- and high-income countries—A qualitative study. PLoS ONE. 2019;14:e0209847. doi: 10.1371/journal.pone.0209847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383.Miljković N., Godman B., van Overbeeke E., Kovačević M., Tsiakitzis K., Apatsidou A., Nikopoulou A., Yubero C.G., Horcajada L.P., Stemer G., et al. Risks in Antibiotic Substitution Following Medicine Shortage: A Health-Care Failure Mode and Effect Analysis of Six European Hospitals. Front. Med. 2020;7 doi: 10.3389/fmed.2020.00157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Miljković N., Godman B., Kovačević M., Polidori P., Tzimis L., Hoppe-Tichy T., Saar M., Antofie I., Horvath L., De Rijdt T., et al. Prospective Risk Assessment of Medicine Shortages in Europe and Israel: Findings and Implications. Front. Pharm. 2020;11 doi: 10.3389/fphar.2020.00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Afriyie D.K., Asare G.A., Amponsah S.K., Godman B. COVID-19 pandemic in resource-poor countries: Challenges, experiences and opportunities in Ghana. J. Infect. Dev. Ctries. 2020;14:838–843. doi: 10.3855/jidc.12909. [DOI] [PubMed] [Google Scholar]
- 386.Björkhem-Bergman L., Andersén-Karlsson E., Laing R., Diogene E., Melien O., Jirlow M., Malmström R.E., Vogler S., Godman B., Gustafsson L.L. Interface management of pharmacotherapy. Joint hospital and primary care drug recommendations. Eur. J. Clin. Pharm. 2013;69:73–78. doi: 10.1007/s00228-013-1497-5. [DOI] [PubMed] [Google Scholar]
- 387.Yoon C.H., Ritchie S.R., Duffy E.J., Thomas M.G., McBride S., Read K., Chen R., Humphrey G. Impact of a smartphone app on prescriber adherence to antibiotic guidelines in adult patients with community acquired pneumonia or urinary tract infections. PLoS ONE. 2019;14:e0211157. doi: 10.1371/journal.pone.0211157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Eriksen J., Gustafsson L.L., Ateva K., Bastholm-Rahmner P., Ovesjo M.L., Jirlow M., Juhasz-Haverinen M., Lärfars G., Malmström R.E., Wettermark B., et al. High adherence to the ‘Wise List’ treatment recommendations in Stockholm: A 15-year retrospective review of a multifaceted approach promoting rational use of medicines. BMJ Open. 2017;7:e014345. doi: 10.1136/bmjopen-2016-014345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Gustafsson L.L., Wettermark B., Godman B., Andersén-Karlsson E., Bergman U., Hasselström J., Hensjö L.-O., Hjemdahl P., Jägre I., Julander M., et al. The ‘Wise List’—A Comprehensive Concept to Select, Communicate and Achieve Adherence to Recommendations of Essential Drugs in Ambulatory Care in Stockholm. Basic Clin. Pharm. Toxicol. 2011;108:224–233. doi: 10.1111/j.1742-7843.2011.00682.x. [DOI] [PubMed] [Google Scholar]
- 390.Klein E.Y., Milkowska-Shibata M., Tseng K.K., Sharland M., Gandra S., Pulcini C., Laxminarayan R. Assessment of WHO antibiotic consumption and access targets in 76 countries, 2000–2015: An analysis of pharmaceutical sales data. Lancet Infect. Dis. 2021;21:107–115. doi: 10.1016/S1473-3099(20)30332-7. [DOI] [PubMed] [Google Scholar]
- 391.Lorencatto F., Charani E., Sevdalis N., Tarrant C., Davey P. Driving sustainable change in antimicrobial prescribing practice: How can social and behavioural sciences help? J. Antimicrob. Chemother. 2018;73:2613–2624. doi: 10.1093/jac/dky222. [DOI] [PubMed] [Google Scholar]
- 392.Sharland M., Pulcini C., Harbarth S., Zeng M., Gandra S., Mathur S., Magrini N. Classifying antibiotics in the WHO Essential Medicines List for optimal use—Be AWaRe. Lancet Infect. Dis. 2018;18:18–20. doi: 10.1016/S1473-3099(17)30724-7. [DOI] [PubMed] [Google Scholar]
- 393.Hsia Y., Sharland M., Jackson C., Wong I.C.K., Magrini N., Bielicki J.A. Consumption of oral antibiotic formulations for young children according to the WHO Access, Watch, Reserve (AWaRe) antibiotic groups: An analysis of sales data from 70 middle-income and high-income countries. Lancet Infect. Dis. 2019;19:67–75. doi: 10.1016/S1473-3099(18)30547-4. [DOI] [PubMed] [Google Scholar]
- 394.Mwita S.J.M., Marwa K., Hamasaki K., Katabalo D., Burger J., Godman B., Ferrario A., Massele A., Ruganuza D. Medicines dispensers’ knowledge on the implementation of an artemisinin-based combination therapy policy for the treatment of uncomplicated malaria in Tanzania. J. Pharm. Health Serv. Res. 2017;8:227–233. doi: 10.1111/jphs.12187. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All sources of information have been referenced.