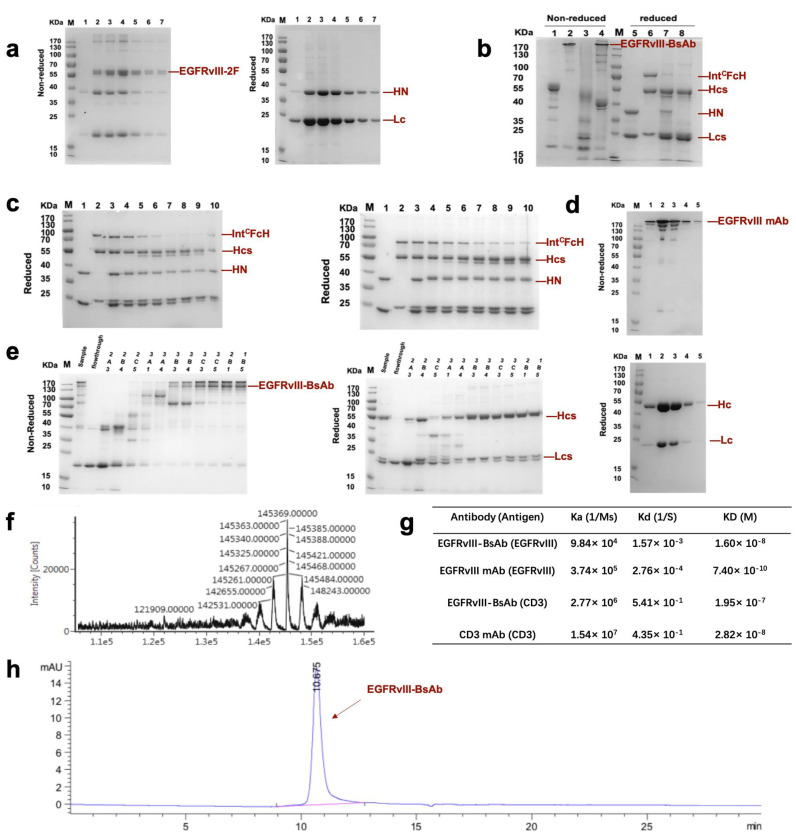
Figure 2.
The generation and characterization of EGFRvIII-BsAb with the BAPTS method. (a) SDS-PAGE analysis of EGFRvIII-2F (fragment B) under nonreduced and reduced conditions. (b) SDS-PAGE analysis of BAPTS reaction. Bands 1,5—EGFRvIII-2F (fragment B); bands 2,6—CD3-3F (fragment A); bands 3,7—BAPTS reaction catalyzed by reduction agent; bands 4,8—complete bispecific antibody obtained by the BAPTS reaction, and followed by oxidization reagent reconstitution. (c) Reduced SDS-PAGE analysis of BAPTS reaction condition optimization. Bands 1–10 represent EGFRvIII-2F, CD3-3F, the mixture of 2F and 3F in the presence of 0 mM, 0.01 mM, 0.05 mM, 0.1 mM, 0.5 mM, 1 mM, 2 mM, and 5 mM reduction agent. (d) Nonreduced (upper) and reduced (lower) SDS-PAGE analysis of purification products of EGFRvIII mAb. (e) Nonreduced (left) and reduced (right) SDS-PAGE analysis of purification products of EGFRvIII-BsAb with an MMC ImpRes Multimodal Chromatography Column. (f) LC/MS analysis of deglycosylated nonreduced BsAb. (g) SPR assay of the dissociation constant of EGFRvIII-BsAb and its control mAbs against recombinant target antigens. (h) SEC-HPLC analysis of EGFRvIII-BsAb.