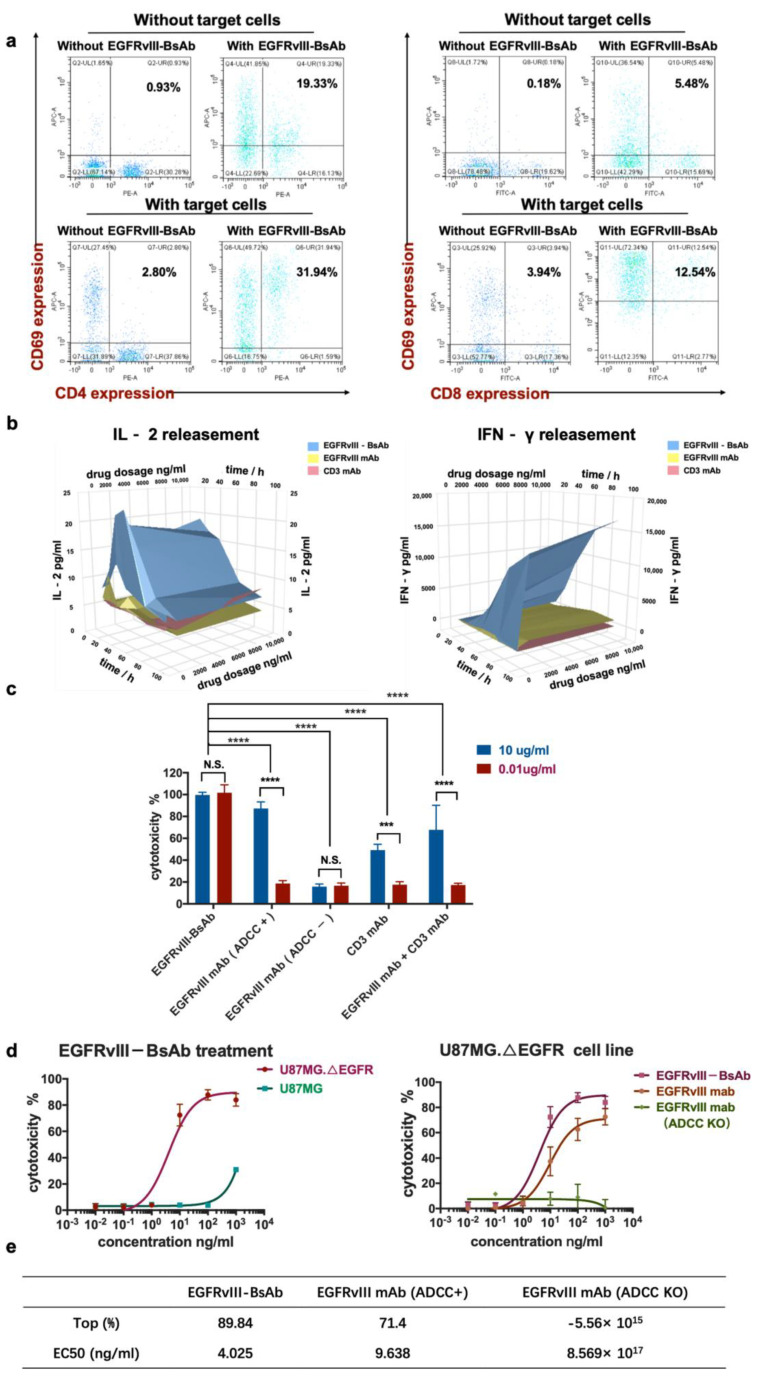
Figure 4.
T-cell activation by EGFRvIII-BsAb. (a) CD69 expression on CD4+ and CD8+ T cells’ surface in response to target tumor cells or the EGFRvIII-BsAb. (b) The secreted levels of IFN-γ and IL-2 in culture supernatant detected after treatment with different EGFRvIII-BsAb concentrations by ELISA. (c) The cytotoxicity measurement of the EGFRvIII-BsAb and its parental antibodies or their combo treatment under low and middle antibody concentration by the LDH method (p < 0.0002 (***), p < 0.0001 (****)). (d) Dose-dependent antitumor activity evaluation of the EGFRvIII-BsAb on U87MG.ΔEGFR or U87MG (left). Dose-dependent antitumor activity evaluation of the EGFRvIII-BsAb compared with the EGFRvIII mAb and the CD3 mAb on U87MG.ΔEGFR cells (right). (e) Maximum cytotoxicity and EC50 comparison among EGFRvIII-BsAb and EGFRvIII mAb or ADCC-attenuated EGFRvIII mAb counterpart. Cytotoxicity measurement by LDH release assay.