Abstract
Background
COVID-19 is a global pandemic caused by the novel coronavirus SARS-CoV-2. Risk factors and prognostic markers of severe disease remain to be fully determined, although some studies have suggested a correlation between abnormal liver function and adverse outcomes. Further studies are needed to investigate this further.
Methods
This retrospective study enrolled patients with a confirmed diagnosis of COVID-19 who were admitted to Kingston Hospital in the UK. Data collected included age, sex, ethnicity, comorbidity profile, biochemical markers of liver function and the acute phase response, and overall outcome.
Results
Between 16 March 2020 and 30 April 2020, a total of 343 patients were admitted to the acute medical team at Kingston Hospital. Excluding those with a history of liver disease, 299 patients had liver function tests performed with abnormalities demonstrated in 44.8% of individuals. Derangement of liver function was associated with greater need for ventilatory support (p<0.001), admission to high dependency unit or intensive care (p<0.001) and increased length of hospital stay (p<0.001). Of note, liver dysfunction was more common in those of non-white ethnicity (p=0.007) and correlated with higher levels of C reactive protein (p=0.01) and ferritin (p<0.001).
Conclusion
Abnormal liver function is associated with a negative outcome among those hospitalised with COVID-19. The cause for this association is unclear, but correlation between abnormal liver function and higher serum levels of acute phase proteins suggest that dysregulation of the immune system in response to SARS-CoV-2 may be contributory.
Keywords: liver, infectious disease, COVID-19
Significance of this study.
What is already known on this topic
It is increasingly well recognised that abnormalities in liver function occur in a proportion of patients with SARS-CoV-2 infection, although the causative mechanisms remain to be fully established.
The frequent use of medications such as lopinavir–ritonavir in many centres across the world have made it difficult to accurately determine the direct impact of the virus on liver function.
What this study adds
Abnormal liver function is common during the course of COVID-19 infection, independent of the use of lopinavir–ritonavir, and there is a strong association with higher serum levels of acute phase proteins.
Patients demonstrating derangement of liver function were more likely to be younger and of non-white ethnicity, and this could not be explained by comorbidity profile alone.
How might it impact on clinical practice in the foreseeable future
Biochemical markers of liver dysfunction may be an important prognostic marker in those hospitalised with COVID-19 and should be actively sought.
Background
COVID-19 is an emerging infectious disease that is caused by the novel coronavirus SARS-CoV-2.1 Since its first description in the city of Wuhan in December 2019,2 the virus has disseminated rapidly across the world, prompting the WHO to declare a global pandemic on 11 March 2020.3 To date, over 45 million people have been infected with the virus worldwide, including over 1 million cases in the UK,4 with significant health, economic and geopolitical consequences.
SARS-CoV-2 is an RNA virus of the coronavirus family, gaining entry into host cells by binding to a transmembrane protein called ACE2.5 6 This enzyme plays an important role in the modulation of the renin–angiotensin system through its carboxypeptidase activity7 and is widely expressed in the human body, including within the lungs, heart and kidneys.8 While SARS-CoV-2 is closely related to other pathogenic coronaviruses such as Middle East Respiratory Syndrome Coronavirus (MERS-CoV),9 10 the scientific community is still learning about this novel virus, including risk factors for severe disease and early biochemical markers that may predict later deterioration.
Recent studies have demonstrated that a significant proportion of individuals with COVID-19 develops abnormalities in liver function during the course of their illness11–15 and some have noted a higher rate of liver injury in those with severe COVID-19.16–18 Of note, many of the studies exploring this subject have been carried out in China,13–18 a country with a relatively high incidence of chronic hepatitis B,19 as well as regular use of lopinavir–ritonavir as part of the treatment for coronavirus,17 20 an agent which can independently exacerbate liver injury.17 20 Therefore, the extent to which conclusions from these studies can be generalised to other populations across the world is unclear.
To investigate further, we carried out a retrospective study in a single centre in the UK. The primary objective was to identify the frequency of liver function derangement among patients admitted to hospital with COVID-19 and explore the association between liver injury and adverse outcomes such as requirement for ventilatory support, admission to intensive care and in-hospital mortality. Our secondary objectives were to identify the risk factors for liver function derangement and correlate markers of liver injury with the serum level of acute phase proteins.
Methods
In this retrospective single centre study, we reviewed all of the patients admitted to the acute medical team at Kingston Hospital between 16 March 2020 and 30 April 2020. A diagnosis of COVID-19 was based on the detection of SARS-COV-2 genetic material from a nasopharyngeal sample. Exclusion criteria included individuals with clinical suspicion of COVID-19 but a negative or indeterminate viral PCR result, patients with a history of liver disease, as well as those admitted directly to the intensive care unit from the Accident and Emergency Department.
Having identified those patients with a positive diagnosis of COVID-19, the following data were collected from their electronic records: age, sex, ethnicity, comorbidities (hypertension, diabetes mellitus, chronic respiratory disease, pre-existing immunosuppression and cardiac disease) and medication history (ACE inhibitors, angiotensin II receptor blockers, antibiotics and lopinavir–ritonavir). Other data included biochemical parameters (C reactive protein, lactate dehydrogenase, ferritin, lymphocyte count, alanine transaminase (ALT), alkaline phosphatase (ALP) and total bilirubin), requirement for ventilatory support (defined as continuous/bi-level positive airway pressure support or endotracheal intubation) and length of hospital stay.
In this study, abnormal liver function was defined as total bilirubin >21 µmol/L, ALT >52 U/L or ALP >130 U/L. With regards to C reactive protein, lactate dehydrogenase and ferritin, values greater than 5 mg/L, 320 U/L and 400 µg/L were defined as abnormal. All of these cut-off values were in line with the normal ranges at our local laboratory.
Statistical analysis
Categorical variables were presented as percentages and compared using the Pearson’s χ2 test or the Fisher’s exact test if the cell counts were small. Continuous variables were expressed as mean±SD and compared using the independent t-test for normally distributed data and the Mann-Whitney U test for non-parametric data. Multivariate logistical regression was used to explore the relationship between liver function derangement and outcome and to calculate an OR, controlling for covariates, including age, sex, ethnicity, comorbidities and antiviral medication. A two-sided p value of<0.05 was considered to be statistically significant. All statistical analyses were performed with SPSS software (V.26.0).
Results
Between 16 March 2020 and 30 April 2020, a total of 343 patients were admitted to the acute medical team at Kingston Hospital and diagnosed with COVID-19 on the same hospital admission. The mean age of patients was 70.2±17.1 years with a predominance of those of white ethnicity (70.6%). It was noted that a significant proportion of individuals was admitted from a residential or nursing home (22.2%). A total of 20 patients had a history of liver disease, while a further 24 did not have any liver function tests performed during the course of their admission. Both groups were subsequently excluded from further data analysis.
Among 299 patients with confirmed COVID-19 and no prior history of liver disease, a total of 88 individuals (29.4%) displayed derangement of liver function on admission, and a further 46 patients (15.4%) developed abnormalities in their liver profile during the course of their hospital stay. Individuals with derangement in their liver function tests were more likely to younger (p<0.001) and of a non-white ethnicity (p=0.007), compared with their counterparts with normal liver biochemistry. There was no statistically significant difference between the two groups in terms of prescription of ACE inhibitors, angiotensin II receptor blockers, antibiotics or lopinavir–ritonavir. Furthermore, the two groups were well matched in the incidence of diabetes, hypertension and chronic respiratory disease, although there was a significant difference in the incidence of pre-existing cardiac disease. Of note, derangement in liver function was strongly associated with elevated serum levels of lactate dehydrogenase (p=0.026), C reactive protein (p=0.01) and ferritin (p<0.001). Baseline characteristics of the patients with COVID-19 are given in table 1.
Table 1.
Baseline characteristics of patients with COVID-19
| Overall (n=299) |
Normal liver function throughout (n=165) |
Abnormal liver function on or during course of admission (n=134) |
P value | |
| Age, years* | 70.3±17.1 | 73.8±16.5 | 65.7±17.0 | <0.001 |
| Male, n (%) | 175 (58.5) | 91 (55.2) | 84 (62.7) | 0.188 |
| Ethnicity, n (%) | ||||
| White | 211 (70.6) | 127 (77.0) | 84 (62.7) | 0.007 |
| Asian | 39 (13.0) | 16 (9.70) | 23 (17.2) | |
| Black African-Caribbean | 12 (4.01) | 5 (3.03) | 7 (5.22) | |
| Mixed | 3 (1.00) | 2 (1.21) | 1 (0.75) | |
| Other | 34 (11.4) | 15 (9.09) | 19 (14.2) | |
| Comorbidities, n (%) | ||||
| Hypertension | 120 (40.1) | 69 (41.8) | 51 (38.1) | 0.510 |
| Diabetes mellitus | 77 (25.8) | 46 (27.9) | 31 (23.1) | 0.351 |
| Chronic respiratory disease* | 68 (22.7) | 41 (24.8) | 27 (20.1) | 0.335 |
| Cardiac disease* | 72 (24.1) | 51 (30.9) | 21 (15.7) | 0.002 |
| Immunosuppression, n (%) | ||||
| Any immunosuppression | 34 (11.4) | 24 (14.5) | 10 (7.46) | 0.055 |
| Corticosteroid | 15 (5.02) | 8 (4.85) | 7 (5.22) | |
| Thiopurine | 2 (0.67) | 1 (0.61) | 1 (0.75) | |
| Methotrexate | 7 (2.34) | 7 (4.24) | 0 (0) | |
| Calcineurin inhibitor | 2 (0.67) | 2 (1.21) | 0 (0) | |
| Mycophenolate | 2 (0.67) | 2 (1.21) | 0 (0) | |
| Biologic therapy | 1 (0.33) | 1 (0.61) | 0 (0) | |
| Haematological malignancy | 11 (3.68) | 8 (4.85) | 3 (2.24) | |
| Recent chemotherapy | 5 (1.67) | 4 (2.42) | 1 (0.75) | |
| Medication during admission, n (%) | ||||
| ACE inhibitor or angiotensin II receptor blocker | 71 (23.7) | 40 (24.2) | 31 (23.1) | 0.823 |
| ACE inhibitor | 41 (13.7) | 25 (15.2) | 16 (11.9) | 0.422 |
| Angiotensin II receptor blocker | 30 (10.0) | 15 (9.09) | 15 (11.2) | 0.547 |
| Antibiotics | 288 (96.3) | 156 (94.5) | 132 (98.5) | 0.07 |
| Lopinavir–ritonavir | 1 (0.33) | 0 (0) | 1 (0.75) | 0.266 |
| Biochemical markers (peak) | ||||
| C reactive protein, mg/L | 175.6±124.7 | 158.6±118.0 | 196.3±129.6 | 0.01 |
| Ferritin, µg/L† | 1875.5±4184.7 | 1083.9±1189.2 | 2542.4±5470.0 | <0.001 |
| LDH, U/L† | 523.5±280.7 | 434.3±148.4 | 572.8±323.9 | 0.026 |
Data are presented as n (%) and mean±SD.
P values refer to the comparison between patients with or without liver function derangement. P values <0.05 are in bold.
*Chronic respiratory disease is defined as a history of obstructive or restrictive lung disease, previous tuberculosis, pulmonary embolism, obstructive sleep apnoea, obesity hypoventilation syndrome or lung malignancy. Cardiac disease is defined as a history of ischaemic heart disease, valvular heart disease, cardiomyopathy, conduction defects or cardiac arrhythmia.
†Note that the total number of patients with values for ferritin and LDH recorded were 119 and 80, respectively.
LDH, lactate dehydrogenase.
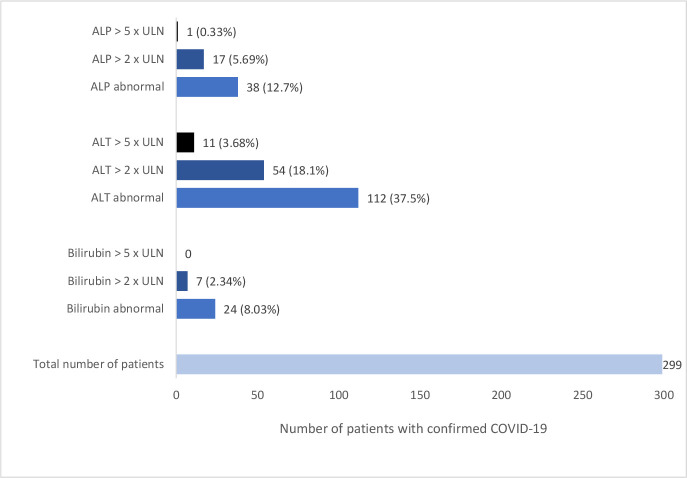
In total, 134 patients (44.8%) developed an abnormality in their liver function either on admission or during the course of their hospital stay. The most common abnormality was an elevation in ALT (112 patients, 37.5%), followed by rise in ALP (38 patients, 12.7%) and total bilirubin (24 patients, 8.03%). Where present, the biochemical changes in liver profile were generally mild, with 54 patients developing an ALT >2 times upper limit of normal and 11 individuals with ALT >5 times upper limit of normal. Maximum values for ALT, ALP and total bilirubin were 752 U/L, 662 U/L and 87 mg/L, respectively. The profile of liver function tests among patients hospitalised with COVID-19 is shown in figure 1.
Figure 1.
Profile of liver function among patients hospitalised with confirmed COVID-19. ULN, upper limit of normal.
With regards to clinical outcome, 48 patients (16.1%) required no supplemental oxygen during their hospital admission while 190 individuals (63.5%) received oxygen via a nasal cannula, face mask or venturi. A total of 61 patients (20.4%) required ventilatory support in the form of continuous/bi-level positive airway pressure ventilation (39 patients) or endotracheal ventilation (22 patients). In total, 64 individuals (21.4%) were admitted to the high dependency unit or intensive care, while the average length of hospital stay was 9.34±11.4 days. Unfortunately, 99 patients died during the same hospital admission, equating to 33.1% of those admitted to hospital and diagnosed with COVID-19. A significant association was found between the development of deranged liver function tests and requirement for ventilatory support (p<0.001, OR: 3.82, CI: 1.88 to 7.75), admission to high dependency or intensive care (p<0.001, OR: 3.62, CI: 1.80 to 7.29), as well as increased length of hospital stay (p<0.001). Of note, there was no significant difference between the two groups in terms of in-hospital mortality (p=0.723). Further details are given in table 2. Additional analysis was carried out to compare the outcome of patients with ALT 1–2 times upper limit of normal versus those with ALT >5 times upper limit of normal. Interestingly, there was no statistically significant difference between the groups in any outcome, including need for ventilatory support (p=0.826), admission to high dependency unit or intensive care (p=0.895) or in-hospital mortality (p=0.328).
Table 2.
Clinical outcomes of patients with COVID-19
| Overall (n=299) | Normal liver function throughout (n=165) |
Abnormal liver function on or during course of admission (n=134) |
P value | |
| Supplemental oxygen via nasal cannula, face mask or venturi, n (%) | 190 (63.5) | 114 (69.1) | 76 (56.7) | 0.074 |
| Ventilatory support, n (%) | 61 (20.4) | 17 (10.3) | 44 (32.8) | <0.001 |
| Admission to high dependency or intensive care, n (%) | 64 (21.4) | 18 (10.9) | 46 (34.3) | <0.001 |
| Length of hospital stay, days | 9.34±11.4 | 7.41±6.20 | 11.7±15.3 | <0.001 |
| In-hospital mortality, n (%) | 99 (33.1) | 62 (37.6) | 37 (27.6) | 0.723 |
Data are presented as n (%) and mean±SD.
P values refer to the comparison between patients with or without liver function derangement. P values <0.05 are in bold.
In multivariate analyses, the following covariates were included: age, ethnicity, sex, comorbidity profile (hypertension, diabetes, chronic respiratory disease, cardiac disease and immunosuppression) and medication (ACE inhibitor, angiotensin II receptor blocker, antibiotics and lopinavir–ritonavir).
To investigate the relationship between ethnicity and clinical outcomes, further analysis was performed. Abnormal liver function was more common in certain ethnic groups with a significantly greater proportion of Asian patients affected compared with those of white ethnicity (59.0% vs 39.8%, p=0.026). Importantly, the two groups were well matched in terms of common comorbidities such as hypertension (p=0.541) and chronic heart disease (p=0.651). Of note, there was no significant difference in liver function abnormality between those of white ethnicity and black Afro-Caribbean patients (p=0.204). In terms of clinical outcome, and having controlled for comorbidity profile, Asian individuals were more likely to be admitted to HDU or ITU compared with Caucasian patients (35.9% vs 17.1%, p=0.006), with a greater risk also demonstrated for those of black Afro-Caribbean ethnicity (41.7% vs 17.1%, p=0.035).
Among 134 patients who displayed alteration in liver biochemistry during their hospital admission, a total of 38 patients (12.7%) underwent imaging of the liver in the form of an ultrasound or cross-sectional imaging. There was normal homogeneous appearance to the liver in the majority of patients (33 cases, 86.8%), with other findings, including hepatic cysts (2 cases, 5.3%), steatosis (2 cases, 5.3%) and distended gallbladder (1 case, 2.6%). Among those with abnormal liver biochemistry, a total of 97 patients survived admission but the majority continued to show deranged liver function at time of discharge (80 patients, 82.5%). Follow-up blood tests were available for 11 of these individuals, with normalisation of liver function in 7 patients (mean time from hospital discharge to follow-up blood test of 34.4 days).
Discussion
COVID-19 is a novel infectious disease that has posed significant challenges for governments and healthcare systems across the world.21 In addition to common symptoms such as fever and dry cough,22 it has become increasingly well recognised that a proportion of patients develop gastrointestinal symptoms such as diarrhoea.23–25 Furthermore, several studies have noted that a significant proportion of patients demonstrate abnormalities in their liver function profile during the course of their illness.11 16 As a novel disease, the long-term complications of the disease are yet to be fully established, including effects on liver function.
In this single centre, retrospective study, 134 patients (44.8%) displayed abnormalities in liver function either on admission or during the course of their hospital stay. The most common abnormality was a rise in ALT (112 patients, 36.5%) with a smaller proportion of patients developing a rise in ALP (38 patients, 12.7%). These results are generally consistent with the results from previous studies, with a rise in serum aminotransferases, the most common biochemical abnormality.11 16
The cause for liver injury in patients with COVID-19 is currently unclear, although it is likely to be multifactorial.26 SARS-CoV-2 enters host cells by binding to the transmembrane enzyme ACE2, followed by interaction with the serine protease TMPRSS2.5 6 It has been demonstrated that the transmembrane receptor ACE2 is expressed in many tissues throughout the body, including the liver, where it is expressed in the highest concentration in cholangiocytes.27 This suggests that abnormal liver function may be caused, at least in part, by a direct cytopathic effect of the virus. Furthermore, several medications used to treat COVID-19 patients are known to cause hepatotoxicity, including the combination drug lopinavir–ritonavir.17 20 One of the strengths of this study is that only a small number of patients received lopinavir–ritonavir during the course of their hospital admission, therefore, allowing a more accurate assessment of the direct impact of coronavirus on liver function to be made. Another potential mode of liver injury in COVID-19 is through dysregulation of the immune system.26 Indeed, studies have suggested that the immune response to SARS-CoV-2 is characterised by high levels of interleukin 628 with greater levels of this pro-inflammatory cytokine in patients who develop severe liver injury.29 In support of this latter hypothesis, it was demonstrated in our study that the development of an abnormal liver profile among hospitalised COVID-19 patients is strongly associated with elevated levels of acute phase reactants, including C reactive protein (p=0.01) and ferritin (p<0.001).
The association between abnormal liver function and severity of disease has been explored in previous studies. These studies have regularly,16–18 but not consistently,30 31 shown derangement in liver function to be associated with severe outcomes. In our study, abnormal liver function was strongly associated with a greater requirement for ventilatory support (p<0.001), admission to the high dependency unit or intensive care (p<0.001), and increased length of hospital stay (p<0.001), having controlled for covariates such as age, sex, ethnicity and comorbidity profile. Interestingly, our data did not show a statistically significant difference in outcome for patients with ALT >5 times upper limit of normal, compared with those with milder derangement, suggesting that there may be a threshold effect, although the sample size for this subanalysis was relatively small. It was noted that individuals who developed abnormal liver function in our study were more likely to be younger (p<0.001) and of a non-white ethnicity (p=0.007), compared with their counterparts with normal liver function. The cause for this association is unclear but may be due to a differential inflammatory response to the virus in certain groups of patients, such as the younger population.
In drawing conclusions from this study, it is important to reflect on some of its limitations, including the exclusion of those individuals who were admitted directly from the Accident and Emergency Department to the intensive care unit. This potentially excluded a significant number of the most severely unwell patients, although it should be noted that many of the patients requiring continuous positive airway pressure ventilation were managed on a medical high dependency unit, rather than intensive care.
Conclusion
In this retrospective study, derangement of liver function tests during the course of COVID-19 infection was common, generally mild and more likely to affect those of a non-white ethnicity. Alteration in liver function is strongly associated with a greater need for ventilatory support, more frequent admissions to intensive care and increased length of hospital stay, although no significant difference in mortality was demonstrated. Further studies are needed to corroborate these findings with the experience from other centres.
Footnotes
Contributors: JL designed the study and collected the data. All authors contributed to the writing of the manuscript and interpretation of the results. All authors read and approved the final manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available upon reasonable request. All data relevant to the study are included in the article.
Ethics statements
Patient consent for publication
Not required.
References
- 1. Zhou P, Yang X-L, Wang X-G, et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020;579:270–3. 10.1038/s41586-020-2012-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wu Z, McGoogan JM. Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China. JAMA 2020;323:1239–42. 10.1001/jama.2020.2648 [DOI] [PubMed] [Google Scholar]
- 3. World Health Organisation . Situation report – 51. Available: https://www.who.int [Accessed 6th Juy 2020].
- 4. UK government data on coronavirus cases and deaths. Available: https://coronavirus-staging.data.gov.uk [Accessed 7th Nov 2020].
- 5. Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020;181:271–80. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Walls AC, Park Y-J, Tortorici MA, et al. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 2020;181:281–92. 10.1016/j.cell.2020.02.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Tikellis C, Thomas MC. Angiotensin-Converting enzyme 2 (ACE2) is a key modulator of the renin angiotensin system in health and disease. Int J Pept 2012;2012:1–8. 10.1155/2012/256294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hamming I, Timens W, Bulthuis MLC, et al. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 2004;203:631–7. 10.1002/path.1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lu R, Zhao X, Li J, et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet 2020;395:565–74. 10.1016/S0140-6736(20)30251-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Andersen KG, Rambaut A, Lipkin WI, et al. The proximal origin of SARS-CoV-2. Nat Med 2020;26:450–2. 10.1038/s41591-020-0820-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Richardson S, Hirsch JS, Narasimhan M, et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the new York City area. JAMA 2020;323:2052. 10.1001/jama.2020.6775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bloom PP, Meyerowitz EA, Reinus Z. Liver Biochemistries in hospitalized patients with COVID‐19. Hepatology 2020;21. [DOI] [PubMed] [Google Scholar]
- 13. Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019-Novel coronavirus (2019-nCoV) pneumonia in Wuhan, China. SSRN Electronic Journal 2020. 10.2139/ssrn.3523861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA 2020;323:1061–9. 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xu X- W, Wu X-X, Jiang X-G, et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ 2020;368:m606. 10.1136/bmj.m606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Xu L, Liu J, Lu M, et al. Liver injury during highly pathogenic human coronavirus infections. Liver Int 2020;40:998–1004. 10.1111/liv.14435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cai Q, Huang D, Yu H, et al. COVID-19: abnormal liver function tests. J Hepatol 2020;73:566–74. 10.1016/j.jhep.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020;395:497–506. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang H, Men P, Xiao Y, et al. Hepatitis B infection in the general population of China: a systematic review and meta-analysis. BMC Infect Dis 2019;19. 10.1186/s12879-019-4428-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fan Z, Chen L, Li J, et al. Clinical features of COVID-19-Related liver damage. SSRN Electronic Journal 2020. 10.2139/ssrn.3546077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. World Health Organisation . Situation report – 167. Available: https://www.who.int [Accessed 6th July 2020].
- 22. Guan W-J, Ni Z-Y, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 2020;382:1708–20. 10.1056/NEJMoa2002032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lin L, Jiang X, Zhang Z, et al. Gastrointestinal symptoms of 95 cases with SARS-CoV-2 infection. Gut 2020;69:997–1001. 10.1136/gutjnl-2020-321013 [DOI] [PubMed] [Google Scholar]
- 24. Redd WD, Zhou JC, Hathorn KE, et al. Prevalence and characteristics of gastrointestinal symptoms in patients with severe acute respiratory syndrome coronavirus 2 infection in the United States: a multicenter cohort study. Gastroenterology 2020;159:765–7. 10.1053/j.gastro.2020.04.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Song Y, Liu P, Shi XL, et al. SARS-CoV-2 induced diarrhoea as onset symptom in patient with COVID-19. Gut 2020;69:1143–4. 10.1136/gutjnl-2020-320891 [DOI] [PubMed] [Google Scholar]
- 26. Schaefer EAK, Arvind A, Bloom PP, et al. Interrelationship between coronavirus infection and liver disease. Clin Liver Dis 2020;15:175–80. 10.1002/cld.967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chai X, Hu L, Zhang Y. Specific ACE2 expression in cholangiocytes may cause liver damage after 2019-nCoV infection. bio-Rxiv 2020. [Google Scholar]
- 28. Blanco-Melo D, Nilsson-Payant BE, Liu W-C, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell 2020;181:1036–45. 10.1016/j.cell.2020.04.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhan K, Liao S, Li J, et al. Risk factors in patients with COVID-19 developing severe liver injury during hospitalisation. Gut 2021;70:628–9. 10.1136/gutjnl-2020-321913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu W, Tao Z-W, Wang L, et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J 2020;133:1032–8. 10.1097/CM9.0000000000000775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med 2020;8:475–81. 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available upon reasonable request. All data relevant to the study are included in the article.