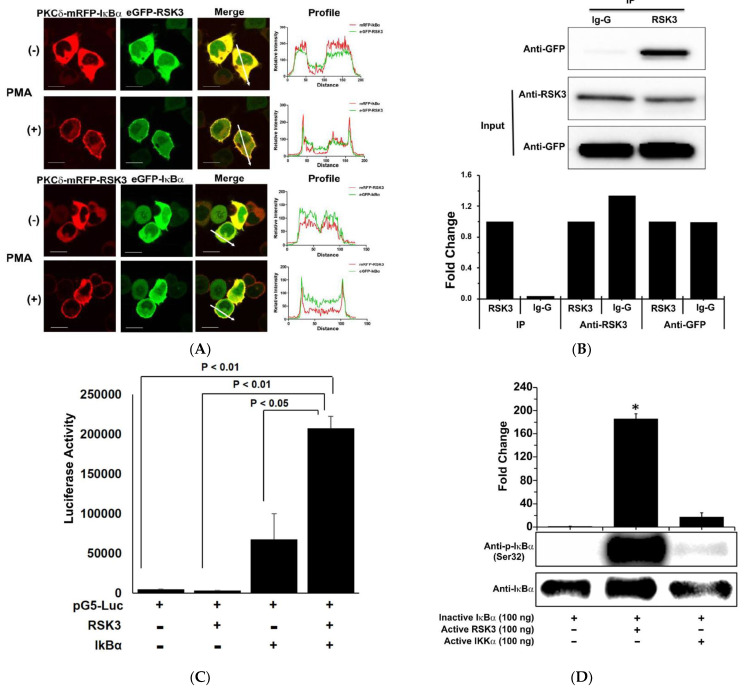
Figure 1.
RSK3 interacts with IκBα and regulates its phosphorylation. (A) Two pairs of vectors, pKCδ-mRFP-IκBα/pAC-GFP-RSK3 or pKCδ-mRFP-RSK3/pAC-GFP-IκBα, were co-transfected into HEK293T cells, and CUPID analysis was performed using a Zeiss 710 Confocal microscope (LSM 710, Carl Zeiss, Germany) after treating with PMA (0.1 nM). The profiles obtained from the images were analyzed in the indicated directions. Scale bar: 10 μm. (B) Myc/His-RSK3 and pAC-GFP-IκBα were co-transfected into HEK293T cells, and RSK3 was immunoprecipitated. Co-immunoprecipitated IκBα was identified by Western blotting using a GFP antibody. Immunoprecipitation was performed three times. Representative results from three experiments are shown. (C) pACT-RSK3 and pBIND-IκBα plasmids were co-transfected into HEK293T cells, and a MTH assay was performed. Luciferase activity indicates the change in relative luminescence units normalized to the negative control. Statistical significance was determined by analysis of variance (Newman–Keuls test). (D) In vitro kinase assays were performed using inactive IκBα (100 ng)/active RSK3 (100 ng) or inactive IkBα (100 ng)/active IKKα (100 ng). Phosphorylated IκBα was detected by Western blot using a phosphospecific (Ser32) IκBα antibody. Representative results from three experiments are shown.