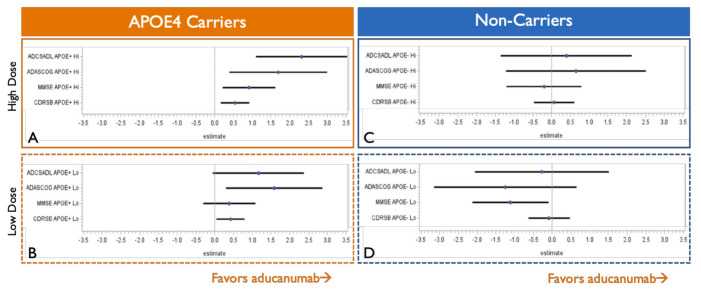
Figure 6.
Carriers of ε4 allele of apolipoprotein E (APOE4) drive the overall efficacy of aducanumab in the EMERGE Phase 3 study. Only a single EMERGE Phase 3 trial showed clinical efficacy of aducanumab. Diagrams adapted from the FDA Advisory Committee Briefing Book illustrate point estimate effects of aducanumab in the EMERGE trial on a functional (Alzheimer’s Disease Cooperative Study—instrumental Activities of Daily Living Inventory (ADCSADL)), cognitive (Alzheimer’s Disease Assessment Scale—Cognitive subscale (ADAS-Cog) and Mini-Mental State Examination (MMSE)), and a composite clinical outcome (Clinical Dementia Rating—Sum of Boxes (CDRSB)). Panels (A) and (B) show effects of high and low doses in APOE4 carriers, and panels (C) and (D) show effects in APOE4 non-carriers, suggesting that the overall efficacy of aducanumab is derived from the APOE4 carrier population with minimal clinical benefits observed in APOE4 non-carriers [68].