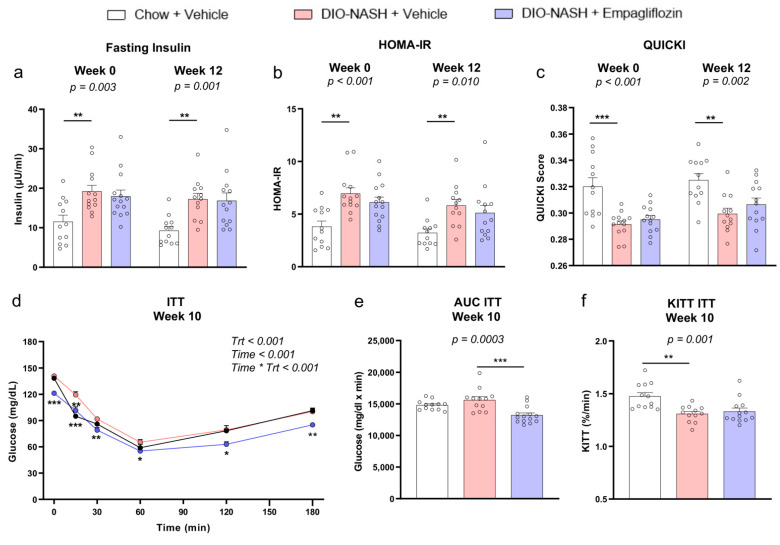
Figure 3.
Empagliflozin does not improve insulin sensitivity in DIO-NASH insulin-resistant mice. (a) Insulin levels after 4 h fasting, (b) HOMA-IR and (c) QUICKI score at week 0 (start of treatment) and week 12 (completion of treatment). (d) Glucose levels, (e) AUC and (f) KITT in the ipITT at week 10. A two-way ANOVA was performed for the ipITT and p-values are reported for Trt, treatment (Chow + vehicle, DIO-NASH + vehicle, DIO-NASH + empagliflozin), Time (minutes of ITT) and their interaction Time * Trt. A one-way ANOVA was performed for all of the other parameters (a–c,e,f). The AUC and p of the ANOVA are reported. By p < 0.05 in Trt * Time for the ipITT and in the ANOVA for the other parameters, post-hoc Dunnett tests were performed in each timepoint in the ipITT, at week 10 in the AUC ITT and KITT ITT and at week 0 and week 12 in the fasting insulin, HOMA-IR and QUICKI and * p < 0.05, ** p < 0.01, *** p < 0.001, respectively, for Chow + vehicle or DIO-NASH + empagliflozin compared with DIO-NASH + vehicle. Data show means ± SEMs. AUC, area under the curve. Ν = 12 for Chow + vehicle and n = 13 for DIO-NASH + vehicle and DIO-NASH + empagliflozin.