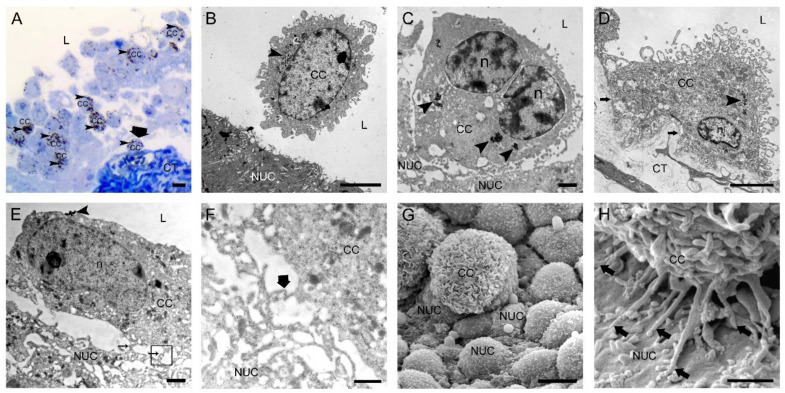
Figure 1.
Two hours after intravesical application of MB49-GFP cancer cells with internalized nanoparticles. (A) Semi-thin section with numerous floating cancer cells (CC) in the lumen and one cancer cell attached to the exposed basal lamina (thick arrow). Toluidine blue staining. (B) Transmission electron micrograph of a floating CC in the lumen and close vicinity to a normal urothelial cell (NUC). (C) Transmission electron micrograph of a CC almost adhered to a less differentiated NUC exposed on the urothelial surface due to previously induced desquamation of superficial urothelial cells. (D) Transmission electron micrograph of a CC tightly attached to the exposed basal lamina (arrows) due to previously induced urothelial cell desquamation. (E) Transmission electron micrograph of a CC with its filopodia (thin arrows) adhered to a NUC at an early step of attachment. The area in the black frame is magnified in F. (F) A higher magnification view of the boxed area (black frame) from E with a contact site of thin protrusions of a CC and a NUC. Thick arrow denotes the position of immunogold labeling of α3β1 integrin at the contact site. (G) Scanning electron micrograph of a CC attached to the urothelial surface with exposed less differentiated NUCs. Note the typically ruffled plasma membrane of a cancer cell. (H) Scanning electron micrograph of a CC attached to a NUC by many tiny filopodia (arrows). Arrowheads (in A–E)—internalized electron-dense nanoparticles in endosomes as a marker of intravesically applied cancer cells; L—lumen of the urinary bladder; n—nucleus; CT—connective tissue. Bars are 10 µm (A,G); 5 µm (B,D); 1 µm (C,E,H); and 600 nm (F).