Figure-3: Delayed treatment with CTLA4-Ig plus Bortezomib inhibits established primary humoral responses in mice immunized with skin transplant.
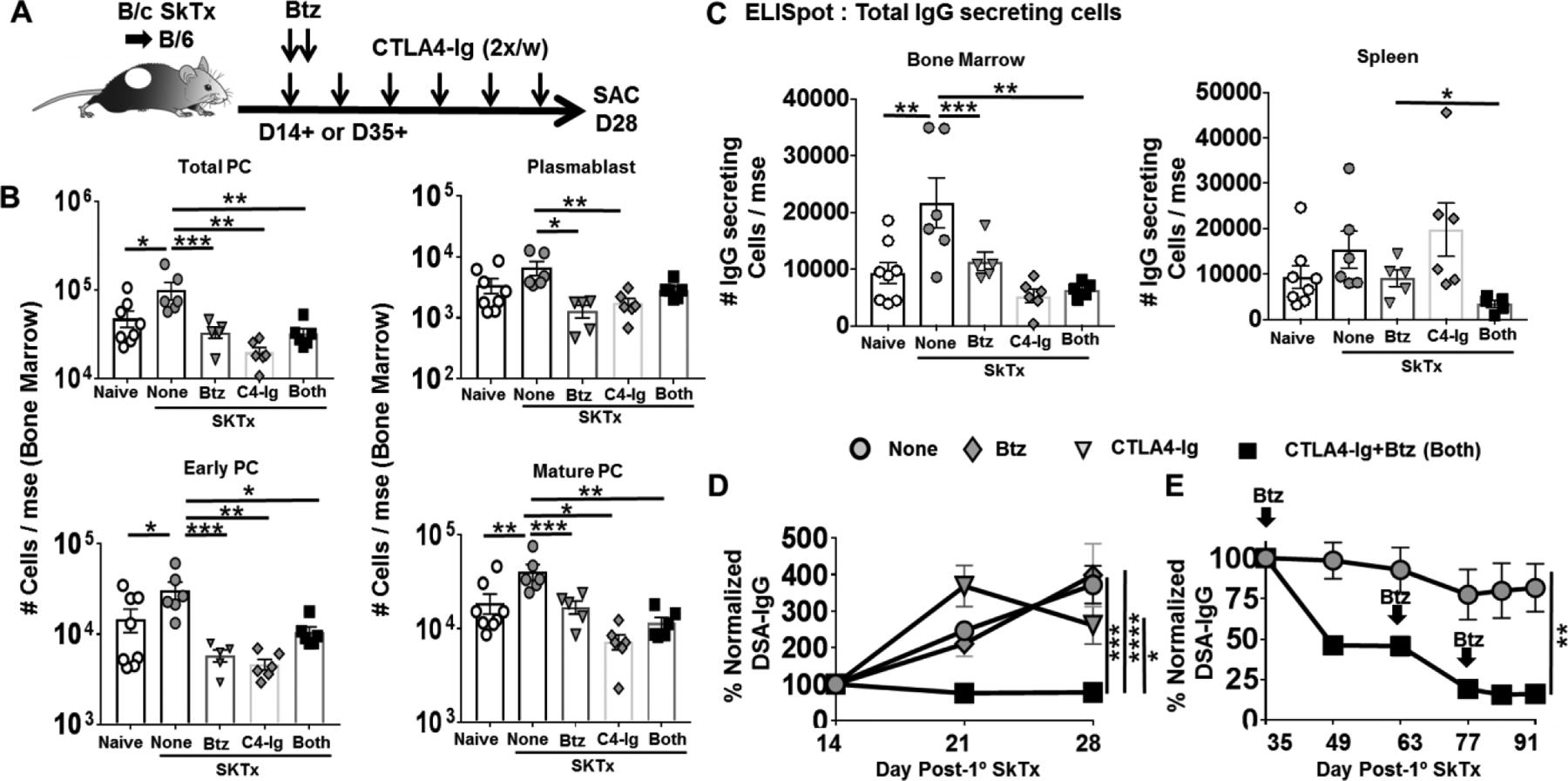
(A) Experimental design. C57BL/6 mice were immunized with BALB/c skin allografts and received no treatment (None), CTLA4-Ig (C4-Ig; from D14), Bortezomib (Btz; D14, D15) or CTLA4-Ig and Bortezomib (Both; from D14 or D35). (B) Quantification of plasma cell (PC) subsets in bone marrow; (C) and total IgG secreting cells in the bone marrow (Left) and spleen (Right) harvested from naïve or D28 post-skin transplant. Y-axis represents total cells retrieved per mouse from naïve, skin transplanted mice not receiving (None) or receiving CTLA4-Ig (POD 14+) or Bortezomib (D14, 15) or Both (from POD14) (N=5–8/group). (D) Anti-BALB/c IgG were assessed from D14–28 post-skin transplant (N=4–6/group). (E) Anti- BALB/c IgG from C57BL/6 WT mice post-skin transplant and received no treatment (None) or CTLA4-Ig and Bortezomib (Both) from POD35+. Repeated Bortezomib treatment on D63 and D77. (N=4–6/group). Each dot represents an individual mouse, pooled from >2 independent experiments. Data are presented as Mean ± SEM and statistically significant differences assessed by one way ANOVA. (*P <0.05) (**P <0.01) (***P <0.001) (****P <0.0001).
