Infections are the second leading cause of death in multiple myeloma (MM), after the disease itself. A large retrospective population-based study revealed a 7-fold higher risk for bacterial and a 10-fold increased risk for viral infections in MM patients compared with population-based controls and showed a particularly high risk shortly after start of therapy.1 Hence, all available measures to curb this complication should be exploited. These include antibiotic prophylaxis in selected cases, antiviral prophylaxis of well-defined risk groups,2 and vaccination against several bacteria and viruses.3 Recently, recommendations for vaccination against various pathogens have been updated.3 In this study, we assessed the type of recommendations provided to MM patients in real-word clinical practice and their compliance with them. Moreover, we surveyed the incidence of infections before and after onset of antimyeloma therapy and patients’ willingness to be vaccinated against coronavirus disease 2019 (COVID-19).
To capture the required information for this survey, we created a questionnaire (See Supplemental Digital Content, File 1, http://links.lww.com/HS/A165) that was specifically designed to assess the vaccination recommendations given to patients from their treatment institutions or physicians and to evaluate their compliance with the advice received. Furthermore, patients were asked about the incidence of infections the year before and the first 6 months after start of therapy and about their willingness to be vaccinated against COVID-19. The first draft was discussed with members of 3 MM support groups. Their comments and suggestions were incorporated in the questionnaire, which was circulated again for comments and suggestions. After approval of the modified questionnaire by the representatives of these patient groups, the instrument was published with an invitation to patients to participate as online tool on the homepages of 31 MM support groups in Germany, Switzerland, and Austria. In addition, 500 hardcopy versions of the questionnaire were mailed directly to patients, thereby reaching out to approximately 2000 patients. Three-hundred thirty-five patients with MM have completed the survey during November 24 and December 15, 2020. Results were analyzed by descriptive statistics and correlation analyses.
Patients’ characteristics are shown in Table 1. Of note, the patients’ profile and their responses did not differ between respondents to the online or printed version of the questionnaire nor between patients from the 3 countries of origin. Patients’ median age was 65 years; the majority (75.4%) received high-dose therapy followed by autologous stem cell transplantation (ASCT) as first-line treatment. Less than 1 quarter (23.2%) had only 1 treatment line, while the majority had been exposed to 2 to 3 (35.1%), or to 4 or more (42.7%) prior treatment lines. Median time from start of first-line therapy to conduction of the survey was 5 years. One-hundred sixty-seven (51.7%) patients reported at least 1 infectious period in the year preceding the diagnosis of MM, 128 (39.6%) patients had 1 to 3, and 39 (12.1%) had 4 or more infectious episodes before start of antimyeloma therapy. During the first 6 months after initiation of first-line therapy, 181 (57.6%) patients reported none, 125 (39.8%) 1 to 3, and 8 (2.5%) 4 or more infectious episodes, respectively.
Table 1.
Patient Characteristics.
| Patient Characteristics (n = 335) | ||
|---|---|---|
| Median age (y; range) | 65 (32–88) | |
| Country (DE/AT/CH; n = 324) | 156 (48.1%)/130 (40.1%)/38 (11.7%) | |
| Gender (male/female; n = 333) | 164 (49.2%)/169 (50.8%) | |
| Prior lines of therapy (n = 305) | ||
| 1 | 70 (23.0%) | |
| 2–3 | 106 (34.8%) | |
| ≥4 | 129 (42.3%) | |
| Median time (y) since start of first-line therapy (range) | 4.96 (0.04–27.97) | |
| ASCT (yes/no; n = 325) | 245 (75.4%)/80 (24.6%) | |
| Infections During | 12 mo Before Start of Therapy, n = 322 | 6 mo After Start of Therapy, n = 314 |
| None | 155 (48.1%) | 181 (57.6%) |
| 1–3 | 128 (39.8%) | 125 (39.8%) |
| ≥4 | 39 (12.1%) | 8 (2.5%) |
ASCT = autologous stem cell transplantation; AT = Austria; CH = Switzerland; DE = Germany.
Recently issued recommendations for vaccination of patients with MM are shown in Supplemental Digital Content, Tables 1–3, http://links.lww.com/HS/A165. The number of patients who received recommendations from their treatment centers and physicians for the various vaccinations and the proportion of patients who actually received the vaccine is shown in Figure 1. The vaccination recommendations varied widely depending on the type of vaccine, with influenza being the most frequently recommended vaccination (77.0%), followed by pneumococci (65.4%), diphtheria, tetanus, pertussis (DTP) (40.6%), hepatitis B (28.1%), and herpes zoster (28.1%), followed by tick borne encephalitis (TBE), hepatitis A, and mumps-measles-rubella (MMR). Thus, physician’s compliance with the recommendations for vaccination was variable but reflected clinical priority. Patients’ compliance with their physicians’ advice was remarkably high with good compliance rates (greater than 80%) for influenza, pneumococci, hepatitis B, and DTP. The rates of recommendations for influenza and pneumococci were higher than reported in a previous study for US myeloma patients4 and supported by clinical experience and data showing that upper and lower respiratory tract infections are the most frequently observed infectious complications in patients with MM.1 Upper respiratory tract infections disturb the complex equilibrium between commensal bacteria and viruses and facilitate the development of lower respiratory tract bacterial and viral infections,5 underlining the need for applying the available prevention strategies. These considerations are supported by the data of this survey that showed a high incidence of infectious episodes in the year before start of antimyeloma therapy, and during the first 6 months after initiation of treatment, with the latter being due to myeloma-induced immune suppression and toxicities of myeloma therapy.
Figure 1.
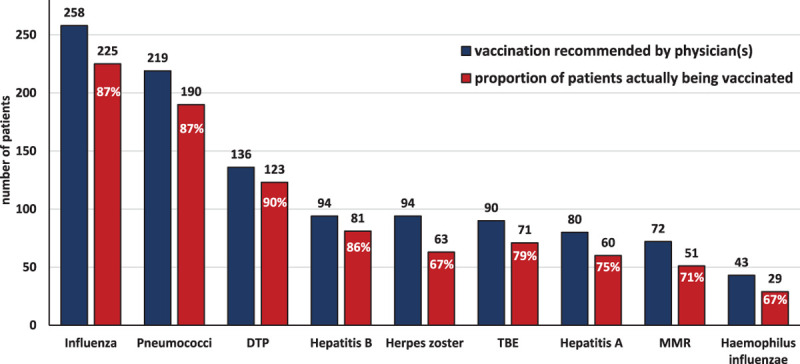
Number of patients who received recommendations for a specific vaccine and proportion of patients who complied with the given advice. DTP = diphtheria, tetanus, pertussis; MMR = measles, mumps, rubella; TBE = tick borne encephalitis.
The high percentage (75.4%) of patients with prior ASCT seems to explain that DTP and MMR vaccinations were recommended to many patients as several of them may lose anti-infective immunity after high dose therapy. Our data show that this advice to get vaccinated with these vaccines was readily taken up by the majority of patients.
Vaccination with varicella zoster virus (VZV) vaccine was recommended to less than one-third of patients, which was lower than expected, since 2 studies in patients with hematological malignancies employing either the inactivated varicella zoster vaccine6 or the preferred adjuvanted recombinant zoster glycoprotein E vaccine7 showed substantial vaccine efficacy of 77.7% and 87.2% with the former and latter product, respectively. Clinicians are likely aware that VZV vaccination does not provide complete protection against VZV reactivations in patients particularly on treatment with proteasome inhibitors, which suppress the priming of naive T cells against viral antigens thereby increasing the susceptibility to viral infections.8 Daratumumab may also be associated with increased risk for reactivation of herpes viruses (herpes simplex virus, VZV, and cytomegalovirus) due to depletion of natural killer cells and possible other perturbations of antiviral defense mechanisms.9 Those patients should, irrespective of their vaccination status, receive anti-VZV prophylaxis.10
Vaccination against hepatitis B and A was proposed to 28.1% and 23.9% of patients, respectively. These lower numbers may be due to low exposure as these vaccines are recommended for MM patients travelling to areas of high endemicity, and to patients with behavioral and/or occupational exposure.3 Furthermore, hepatitis B vaccination should be considered only in patients who are negative for hepatitis B surface antigen and anti-hepatitis B core antigen antibodies. Those with one or both tests positive should be studied for the presence of hepatitis B DNA, and if positive should be treated with one of the newer oral antinucleoside analogs.11 Reactivation of hepatitis B infection has been reported to occur early after start but also after prolonged treatment and in patients exposed to ASCT.
Vaccination with haemophilus influenzae type B (HiB) was recommended to 12.8% of patients only, a figure that is below expectations given the fact that only 67.4% complied with this advice. More than 50% of patients lack protective antihaemophilus influenza type B antibodies and serum bactericidal activity against HiB was found to be absent in 70% of patients.12 Hence, awareness about the potential benefits of HiB vaccination should be raised both on the side of care provider and in MM patients.
TBE is endemic in several parts of the surveyed countries, which likely explains that slightly more than one-fourth (26.9%) of patients were advised to be vaccinated and that close to 80% of patients followed this recommendation. A recent report in patients with hematological malignancies after ASCT or allogeneic-SCT revealed seropositivity in 77% and 80% of patients, respectively, scheduled for 4 doses of a TBE vaccine.13
The relatively high acceptance rate of vaccinations by informed patients participating in this survey was manifested by their eager willingness to get vaccinated with a severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vaccine (82.5%), which is exemplary as compared with the general population of the United States and of Germany (the latter likely changing over time, while more have been vaccinated with good tolerance).14 Possible explanations for this proactive attitude in MM patients may be their higher individual risks for infections, high mortality rate in case of a SARS-CoV-2 infection,15 greater interest in relevant developments in medicine, closer contacts with health care personnel with frequent advise to get vaccinated, and being diagnosed with a serious disease, facts that increase the willingness to accept preventive measures and treatment.
An inherent limitation of surveys, among others, is that only active and empowered patients are responding, leaving it open, whether the outcome would have been different in the nonresponding patient population, and thus possibly not representative of the entire patient population, Usually, elderly people are less likely to participate in surveys, which is also reflected by the median age of the respondents of 65 years in this study. Nevertheless the comparison of responses between patients aged ≥70 years with those obtained from the younger participants did not reveal any clinically relevant or statistically significant differences. Likewise, no significant associations were noted between individual patient characteristics, duration of disease, number of treatment lines, incidence of infections, and their compliance with vaccination advice. Finally, the vaccination practices observed in this MM patient cohort suggests that awareness of the positive effects of vaccinations should be raised both among treating physicians and patients, a notion especially important in pandemic/COVID-19 times.
Acknowledgments
We thank the willingness of myeloma patients to respond to the questionnaire and the self-help groups for their participation.
Disclosures
HL received research funding from Amgen and Takeda, as well as honoraria for speaker’s bureau/advisory boards from Amgen, Sanofi, Janssen, BMS-Celgene, and Seattle Genetics. ME has received clinical trial funding from Amgen, BMS, Janssen, Takeda; and speaker honoraria from Amgen, BMS, Janssen, Sanofi, and Takeda. AM has no conflicts of interest to disclose.
Source of funding
This work was supported by the Austrian Forum Against Cancer.
Supplementary Material
Footnotes
Supplemental digital content is available for this article.
References
- 1.Blimark C, Holmberg E, Mellqvist UH, et al. Multiple myeloma and infections: a population-based study on 9253 multiple myeloma patients. Haematologica. 2015; 100:107–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Teh BW, Slavin MA, Harrison SJ, et al. Prevention of viral infections in patients with multiple myeloma: the role of antiviral prophylaxis and immunization. Expert Rev Anti Infect Ther. 2015; 13:1325–1336 [DOI] [PubMed] [Google Scholar]
- 3.Ludwig H, Boccadoro M, Moreau P, et al. Recommendations for vaccination in multiple myeloma: a consensus of the European Myeloma Network. Leukemia. 2021; 35:31–44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alemu A, Singh M, Blumberg C, et al. Multiple myeloma vaccination patterns in a large health system: a pilot study. J Patient Cent Res Rev. 2017; 4:53–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bosch AA, Biesbroek G, Trzcinski K, et al. Viral and bacterial interactions in the upper respiratory tract. PLoS Pathog. 2013; 9:e1003057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Winston DJ, Mullane KM, Cornely OA, et al. V212 Protocol 001 Trial Team Inactivated varicella zoster vaccine in autologous haemopoietic stem-cell transplant recipients: an international, multicentre, randomised, double-blind, placebo-controlled trial. Lancet. 2018; 391:2116–2127 [DOI] [PubMed] [Google Scholar]
- 7.Dagnew AF, Ilhan O, Lee WS, et al. Zoster-039 study group Immunogenicity and safety of the adjuvanted recombinant zoster vaccine in adults with haematological malignancies: a phase 3, randomised, clinical trial and post-hoc efficacy analysis. Lancet Infect Dis. 2019; 19:988–1000 [DOI] [PubMed] [Google Scholar]
- 8.Basler M, Lauer C, Beck U, et al. The proteasome inhibitor bortezomib enhances the susceptibility to viral infection. J Immunol. 2009; 183:6145–6150 [DOI] [PubMed] [Google Scholar]
- 9.Nahi H, Chrobok M, Gran C, et al. Infectious complications and NK cell depletion following daratumumab treatment of multiple myeloma. PLoS One. 2019; 14:e0211927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baden LR, Swaminathan S, Angarone M, et al. Prevention and treatment of cancer-related infections, version 2.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016; 14:882–913. [DOI] [PubMed] [Google Scholar]
- 11.EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol. 2017; 67:370–398 [DOI] [PubMed] [Google Scholar]
- 12.Nix EB, Hawdon N, Gravelle S, et al. Risk of invasive Haemophilus influenzae type b (Hib) disease in adults with secondary immunodeficiency in the post-Hib vaccine era. Clin Vaccine Immunol. 2012; 19:766–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Einarsdottir S, Nicklasson M, Veje M, et al. Vaccination against tick-borne encephalitis (TBE) after autologous and allogeneic stem cell transplantation. Vaccine. 2021; 39:1035–1038 [DOI] [PubMed] [Google Scholar]
- 14.Kantar Deutschland. https://www.kantardeutschland.de/ploads/20201119_Kantar_Studie_Corona_Impfung_Chartset.pdf. Accessed December 4, 2020
- 15.Martínez-López J, Mateos MV, Encinas C, et al. Multiple myeloma and SARS-CoV-2 infection: clinical characteristics and prognostic factors of inpatient mortality. Blood Cancer J. 2020; 10:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


