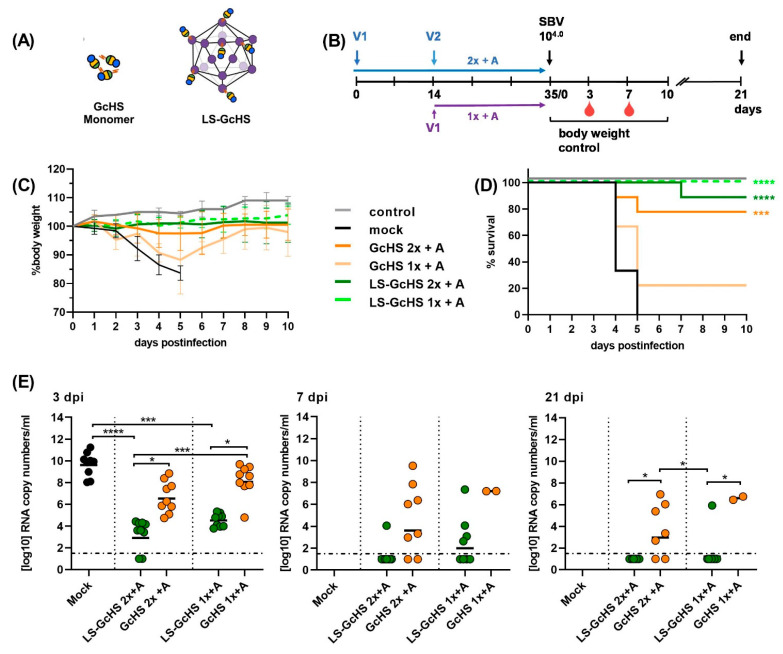
Figure 6.
IFNAR-/- mouse trial #3. Evaluation of the C1-produced GcHS domain and the protective efficacy of the monomeric and LS-conjugated antigen. (A) Schematic of vaccines used for the immunizations in this trial; (B) Experimental scheduling; (C) Body weight development after challenge infection, each line represents the mean value of the respective group with SD; (D) Survival curves after challenge infection. One animal in group LS-GcHS vaccinated twice (2x + A) accidentally died during blood sampling in a manner unrelated to SBV infection; (E) SBV RNA detected by RT-qPCR in EDTA blood samples of surviving animals in each group at 3, 7 or 21 dpi, respectively. Dashed lines indicate the detection limit of the RT-qPCR assay. Samples of animals that succumbed to infection or had to be euthanized prior to or on the respective sampling day were not included. In (E) statistical analysis was performed using the Kruskal–Wallis test followed by Dunn´s test for comparisons between individual groups. p values < 0.05 were considered significant (* p < 0.05; ** p < 0.01; *** p < 0.001; **** p < 0.0001). Only significant differences between groups are labeled. Differences that are not significant (p > 0.05) are not separately indicated. In (D) significant differences compared to the mock control were calculated using the Mantel–Cox test (* 0.0332; ** 0.0021; *** 0.0002; **** <0.0001). Comparisons between all groups against each other are not indicated but are shown in Table S2.