INTRODUCTION
Li-Fraumeni syndrome (LFS) is a rare cancer predisposition syndrome characterized by germline pathogenic variants in the TP53 tumor suppressor gene.1 LFS is characterized by a high risk of developing many tumor types including sarcomas, leukemia, CNS tumors, breast cancer, and adrenocortical carcinoma.2 Lung cancer risk is also part of the LFS tumor spectrum, with a frequency of 2%-7%,3 although the clinical phenotype is less well-characterized. In a 2017 cohort study, the estimated risk for lung cancer in patients with LFS was approximately 22%, with diagnoses nearly always occurring in adulthood.4 An additional two studies have been published that include characterization of lung cancer in LFS, although neither of these studies describe the modality of detection in detail. LFS cancer surveillance protocols include whole-body magnetic resonance imaging (WBMRI) and other targeted screening (ie, breast magnetic resonance imaging [MRI], brain MRI, and upper and lower endoscopy). This intensive approach allows for earlier detection and improved survival in comparison with detection of cancer solely on the basis of symptom detection.5,6 Although computed tomographic scans and positron emission tomographic scans have well-defined roles for screening in the general population7,8 and staging9 lung cancer, respectively, the use and effectiveness of MRI to detect lung cancer in the setting of LFS is less well-established. Studies have indicated that WBMRI can identify non–small-cell lung cancer (NSCLC) in patients with LFS, but these reports have not included review of serial imaging to determine when in development these lesions came to attention or the clinical outcomes of these tumors.4,5,10
Independent and separate from LFS, lung cancer is the leading cause of cancer mortality in the United States.11 Eighty-five percent of lung cancer is non–small-cell type (NSCLC), of which adenocarcinoma is the dominant histology.11,12 The median age at diagnosis is 70 years.11 Tobacco smoking is the greatest risk factor for NSCLC development, although radon exposure, family history, and cancer predisposition syndromes contribute to a lesser degree.11-14
Our study aim is to describe the clinical phenotypes and imaging findings of patients diagnosed with NSCLC in the setting of LFS. The knowledge generated from this report serves to educate the general oncology community and add to the growing body of data on NSCLC in LFS.
METHODS
This was an institutional review board–approved single-institution case series of patients with LFS and a history of lung cancer, identified via an internal LFS patient registry (Huntsman Cancer Institute, University of Utah), which included 91 adult patients (age ≥ 18 years) at the time of analysis. Cancer genetics and medical oncology personnel reviewed the patients' electronic medical records and gathered pertinent clinical history. A thoracic subspecialized radiologist reviewed the patients' available imaging. Volume-doubling times (VDTs) of lung cancers were calculated when sufficient imaging was available. Somatic mutation status was determined via next-generation sequencing (NGS) or polymerase chain reaction depending on standard of care at time of diagnosis. Germline TP53 variant identification and classification was performed at commercial, Clinical Laboratory Improvement Act–approved laboratories.
All patient data were collected as part of an institutional review board–approved study through the department of genetics at Huntsman Cancer Institute through the University of Utah. We obtained informed consent from the five patients and/or their living relatives to publish the relevant information and images.
RESULTS
Six of 91 patients (6.6%) in our LFS cohort were diagnosed with lung cancer. Table 1 summarizes the clinical information, family history, somatic and germline variant status, imaging findings, and treatment history for five patients (one patient's record was not available). All patients were diagnosed with stage I-III adenocarcinoma, 80% were never-smokers, 60% were female, and the median age at diagnosis was 48 years. All patients had previous or concurrent cancer diagnoses, and none had received prior chest irradiation for treatment. Patients 1, 2, and 5 had family histories meeting classic LFS diagnostic criteria. Each patient had a unique TP53 germline pathogenic variant. Forty percent of patients had common EGFR mutations, and two of the cases did not have testing in the setting of stage I disease. Pretreatment imaging for one patient was unavailable for review. Two patients had cross-sectional imaging of chest with a sufficient time interval to calculate a VDT.
TABLE 1.
Clinical History and Imaging Findings
DISCUSSION
In our single-institution case series, the clinical phenotypes of patients diagnosed with lung cancer in the setting of LFS were similar to those in the general never-smoking NSCLC population, with the exception of earlier stage at presentation.15,16 Similar to the clinical phenotype identified in other case series,17 our patients were younger, were more often female, and had a high frequency of EGFR mutations. In contrast to the typical presentation of never-smokers' EGFR-mutated lung cancer, all patients with LFS in this study were diagnosed with potentially curative stage I-III disease, reinforcing the importance of close surveillance and cancer screening among patients with LFS.
Emerging evidence demonstrates patients with LFS with advanced lung cancer have a higher frequency of EGFR driver mutations than the general NSCLC population along with a high somatic mutation burden.17-19 Current national guidelines recommend NGS testing in all patients with metastatic lung adenocarcinoma, and the NCCN has updated its recommendations to consider EGFR molecular testing in surgically resected stage IA-IIIB cases.20 Given our study findings of a 40% rate of EGFR mutations in the early-stage patients who received molecular testing, oncologists should consider NGS testing in all patients with LFS lung cancer, regardless of stage. This is relevant in light of recently published data showing a significant disease-free survival benefit when adjuvant osimertinib is used in surgically resected early-stage (IB-IIIA) patients with EGFR variants.21 Osimertinib is now US Food and Drug Administration–approved for this indication. Additionally, published data suggest that patients with co-occurring somatic EGFR and germline TP53 pathogenic variants have expected responsiveness to EGFR-targeted therapy,17 in stark contrast to the poorer outcomes seen in those with co-occurring TP53 somatic variants.22
Another observation of note includes the variability in treatment recommendations among patients with LFS with lung cancer in this series. For example, a stage I patient was treated with cryoablation instead of stereotactic radiation because of concerns for future development of a radiation-induced malignancy. Although this concern is logical, given the role of p53 in DNA repair, clinical evidence is mixed. Several recent publications have suggested that the risk for radiation-induced secondary cancers may not be sufficient to warrant forgoing standard-of-care treatment in patients with LFS.23-25 It is therefore reasonable to administer radiation therapy if appropriate within the context of an LFS patient’s malignancy. All available evidence suggests that patients with lung cancer with LFS respond to standard systemic therapy as expected, so they should be treated according to the standard of care for general patients with NSCLC.
The imaging appearance of the lung adenocarcinomas of the patients with LFS in this case series were typical of that seen in the general population, and although the VDT could only be calculated for two of the patients, the resulting VDTs (247 and 495 days) were also typical of lung adenocarcinoma26 (Fig 1). Upon reviewing the patients' imaging portfolios, one instance was identified wherein the lung cancer was subtly detectible in retrospect on a WBMRI but not described in the radiologist's report, which led to a delay in care. Detection of pulmonary abnormalities is challenging on WBMRI because of the limited sequences, large field of view, and poor spatial resolution (Fig 2). However, reports in the literature describe optimization of MRI for lung cancer screening, capable of imaging pulmonary nodules as small as 4 mm in size.27 A dedicated chest MRI, acquired at the same time as the WBMRI, can provide a more sensitive imaging approach for lung cancer screening, without requiring a separate scan.
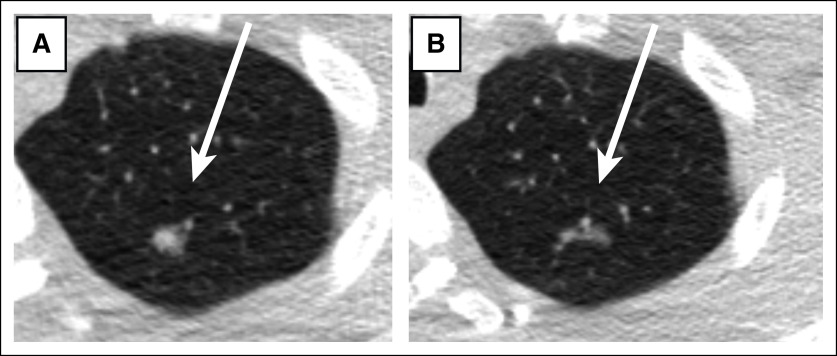
FIG 1.
Adenocarcinoma in a patient with Li-Fraumeni syndrome with classic imaging appearance. A 15 × 6 × 6 mm nodule in the left lung apex with (A) solid and (B) subsolid components.
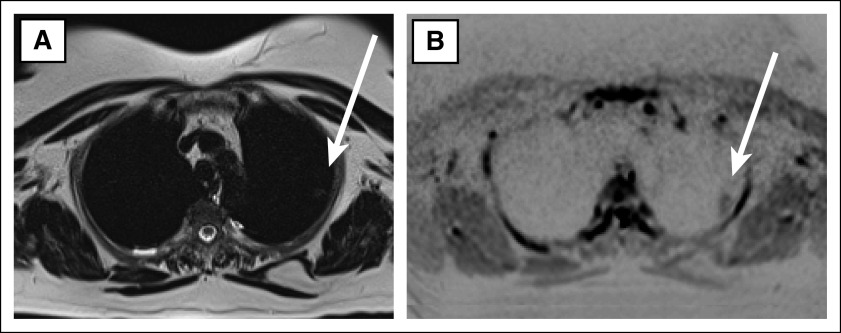
FIG 2.
Adenocarcinoma in a patient with Li-Fraumeni syndrome in the left upper lung on whole-body MRI: (A) axial T2-HASTE and (B) axial DWI_TRACE.
Additionally, we identified a few instances of delayed treatment because of the complexities of caring for patients with a rare cancer predisposition syndrome who receive their care at multiple institutions, including surveillance and diagnostic imaging. Providing context for cancer risk, specifically a detailed cancer history in this cancer-predisposed population, is extremely valuable to the interpreting radiologist. Periodic multidisciplinary conferences for patients with LFS create an opportunity to consolidate information among multiple providers and institutions as well as enable a radiologist to review the patient's imaging, clarify relevant findings, and recommend any further imaging.
Our study has several limitations including small sample size, the lack of comprehensive somatic mutation testing, and the use of variable imaging modalities. Despite these limitations, an additional six cases of lung cancer in the LFS population is a significant contribution to the existing body of literature. Comprehensive reviews of screening WBMRI for properly evaluating lung parenchyma in the LFS population are sparse, and we demonstrate the need for a larger, in-depth analysis. Future work should focus on expanding these results through a multicenter review to corroborate these findings and update lung cancer screening recommendations as needed in the high-risk LFS population.
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
SUPPORT
Support for the use of Genetic Counseling Shared Resources by National Cancer Institute under award number P30CA042014 to the Huntsman Cancer Institute and the Huntsman Cancer Foundation. J.S. is supported by Hyundai Hope on Wheels, Soccer for Hope Foundation, Li-Fraumeni Syndrome Association, Kneaders Bakery & Café Hope Campaign, 5 For The Fight (Qualtrics), and the Elephant p53 (EP53) Program funded through Huntsman Cancer Institute by the State of Utah.
AUTHOR CONTRIBUTIONS
Conception and design: Kathleen Kerrigan, Jennie Vagher, Wendy Kohlmann, Luke Maese
Financial support: Kathleen Kerrigan
Administrative support: Kathleen Kerrigan, Jo Anson
Provision of study materials or patients: Kathleen Kerrigan
Collection and assembly of data: Kathleen Kerrigan, Jennie Vagher, Wendy Kohlmann, Anne Naumer, Jo Anson, Sara Low, Luke Maese
Data analysis and interpretation: Kathleen Kerrigan, Jessica Chan, Jennie Vagher, Wendy Kohlmann, Joshua Schiffman, Luke Maese
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/po/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Kathleen Kerrigan
Stock and Other Ownership Interests: Bristol-Myers Squibb
Travel, Accommodations, Expenses: Flatiron Health
Jennie Vagher
Consulting or Advisory Role: InVitae
Wendy Kohlmann
Employment: BioFire Diagnostics
Joshua Schiffman
Employment: PEEL Therapeutics
Leadership: PEEL Therapeutics
Stock and Other Ownership Interests: ItRunsInMyFamily.com, PEEL Therapeutics
Honoraria: Affymetrix
Consulting or Advisory Role: N-of-One, Fabric Genomics
Luke Maese
Honoraria: Jazz Pharmaceuticals
Consulting or Advisory Role: Jazz Pharmaceuticals
No other potential conflicts of interest were reported.
REFERENCES
- 1.Malkin D, Li FP, Strong LC, et al. : Germ line p53 mutations in a familial syndrome of breast cancer, sarcomas, and other neoplasms. Science 250:1233-1238, 1990 [DOI] [PubMed] [Google Scholar]
- 2.Li FP, Fraumeni JF Jr: Soft-tissue sarcomas, breast cancer, and other neoplasms. A familial syndrome? Ann Intern Med 71:747-752, 1969 [DOI] [PubMed] [Google Scholar]
- 3.McBride KA, Ballinger ML, Killick E, et al. : Li-Fraumeni syndrome: Cancer risk assessment and clinical management. Nat Rev Clin Oncol 11:260-271, 2014 [DOI] [PubMed] [Google Scholar]
- 4.Caron O, Frebourg T, Benusiglio PR, et al. : Lung adenocarcinoma as part of the Li-Fraumeni syndrome spectrum: Preliminary data of the LIFSCREEN randomized clinical trial. JAMA Oncol 3:1736-1737, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ballinger ML, Best A, Mai PL, et al. : Baseline surveillance in Li-Fraumeni syndrome using whole-body magnetic resonance imaging: A meta-analysis [published correction appears in JAMA Oncol 2018 Apr 1;4(4):590]. JAMA Oncol 3:1634-1639, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kratz CP, Achatz MI, Brugières L, et al. : Cancer screening recommendations for individuals with Li-Fraumeni syndrome. Clin Cancer Res 23:e38-e45, 2017 [DOI] [PubMed] [Google Scholar]
- 7.de Koning HJ, van der Aalst MC, de Jong PA: Reduced lung-cancer mortality with volume CT screening in a randomized trial. N Engl J Med 382:503-513, 2020 [DOI] [PubMed] [Google Scholar]
- 8.The National Lung Cancer Screening Trial Research Team : Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med 365:395-409, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lardinois D, Weder W, Hany TF, et al. : Staging of non–small-cell lung cancer with integrated positron-emission tomography and computed tomography. N Engl J Med 348:2500-2507, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Villani A, Shore A, Wasserman JD, et al. : Biochemical and imaging surveillance in germline TP53 mutation carriers with Li-Fraumeni syndrome: 11 Year follow-up of a prospective observational study. Lancet Oncol 17:1295-1305, 2016 [DOI] [PubMed] [Google Scholar]
- 11.American Cancer Society : Cancer Facts & Figures 2020. Atlanta, GA, American Cancer Society, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ettinger DS, Wood DE, Aggarwal C, et al. : NCCN guidelines insights: Non-small cell lung cancer, version 1.2020. J Natl Compr Canc Netw 17:1464-1472, 2019 [DOI] [PubMed] [Google Scholar]
- 13.Carr SR, Akerley W, Cannon-Albright LA: Genetic contribution to nonsquamous, non-small cell lung cancer in nonsmokers. J Thorac Oncol 13:938-945, 2018 [DOI] [PubMed] [Google Scholar]
- 14.Cannon-Albright LA, Carr SR, Akerley W: Population-based relative risks for lung cancer based on complete family history of lung cancer. J Thorac Oncol 14:1184-1191, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wakelee HA, Chang ET, Gomez SL, et al. : Lung cancer incidence in never smokers. J Clin Oncol 25:472-478, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rudin CM, Avila-Tang E, Harris CC, et al. : Lung cancer in never smokers: Molecular profiles and therapeutic implications. Clin Cancer Res 15:5646-5661, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mezquita L, Jove M, Nadal E, et al. : High prevalence of somatic oncogenic driver alterations in patients with NSCLC and Li-Fraumeni syndrome. J Thorac Oncol 15:1232-1239, 2020 [DOI] [PubMed] [Google Scholar]
- 18.Ricordel C, Labalette-Tiercin M, Lespagnol A, et al. : EFGR-mutant lung adenocarcinoma and Li-Fraumeni syndrome: Report of two cases and review of the literature. Lung Cancer 87:80-84, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Michalarea V, Calcasola M, Cane P, et al. : EGFR-mutated lung cancer in Li-Fraumeni syndrome. Lung Cancer 85:485-487, 2014 [DOI] [PubMed] [Google Scholar]
- 20.Ballinger ML, Best A, Mai PL, et al. : Baseline surveillance in Li-Fraumeni syndrome using whole-body magnetic resonance imaging: A meta-analysis. JAMA Oncol 3:1634-1639, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu YL, Tsuboi M, He J, et al. : Osimertinib in resected EGFR-mutated non-small-cell lung cancer. N Engl J Med 383:1711-1723, 2020 [DOI] [PubMed] [Google Scholar]
- 22.Canale M, Petracci E, Delmonte A, et al. : Impact of TP53 mutations on outcome in EGFR-mutated patients treated with first-line tyrosine kinase inhibitors. Clin Cancer Res 23:2195-2202, 2017 [DOI] [PubMed] [Google Scholar]
- 23.Hendrickson PG, Luo Y, Kohlmann W, et al. : Radiation therapy and secondary malignancy in Li-Fraumeni syndrome: A hereditary cancer registry study. Cancer Med 9:7954-7963, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petry V, Bonadio RC, Cagnacci AQC, et al. : Radiotherapy-induced malignancies in breast cancer patients with TP53 pathogenic germline variants (Li-Fraumeni syndrome). Fam Cancer 19:47-53, 2020 [DOI] [PubMed] [Google Scholar]
- 25.Le AN, Harton J, Desai H, et al. : Frequency of radiation-induced malignancies post-adjuvant radiotherapy for breast cancer in patients with Li-Fraumeni syndrome. Breast Cancer Res Treat 181:181-188, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park S, Lee SM, Kim S, et al. : Volume doubling times of lung adenocarcinomas: Correlation with predominant histologic subtypes and prognosis. Radiology 295:703-712, 2020 [DOI] [PubMed] [Google Scholar]
- 27.Wang YX, Lo GG, Yuan J, et al. : Magnetic resonance imaging for lung cancer screen. J Thorac Dis 6:1340-1348, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]