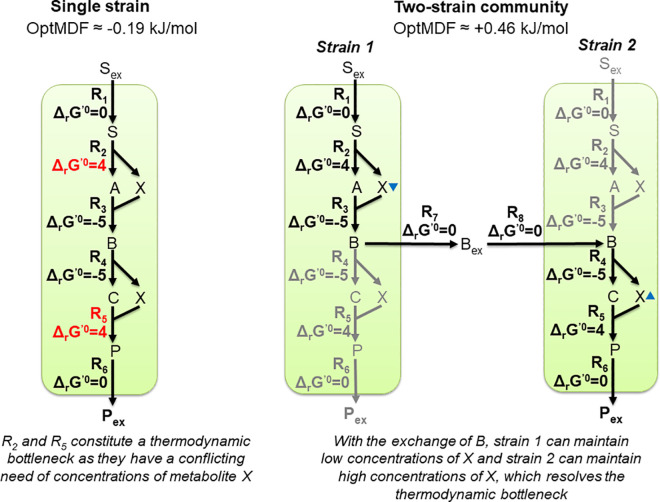
Fig 2. Example illustrating how division of labor may lead to a thermodynamic advantage in the production of a target metabolite.
In the left, a metabolic pathway in a cell is considered that synthesizes the target product P. The red values indicate positive values for the standard Gibbs free energy change ( [in kJ/mol]) and thus potential thermodynamic bottlenecks. With an allowed concentration range from 1 M to 10 M for all metabolites except for Pex, where a minimum concentration of 5 M was assumed to consider product synthesis under high external product concentrations, a negative optimal MDF (OptMDF) value would follow, indicating thermodynamic infeasibility of product synthesis in the single strain. In the two-strain community (right), the pathway is divided and an exchange of metabolite B introduced. With this, individual concentrations of metabolite X can be adjusted in the two strains by which thermodynamic feasibility (a positive OptMDF) of the overall transformation is achieved (the blue triangles indicate the direction of the concentrations of X (high/low) when maximizing the driving force). Black arrows in the two-strain solution indicate active and grey arrows inactive reactions.