Abstract
Rhododendron (Ericaceae) extracts contain flavonoids, chromones, terpenoids, steroids, and essential oils and are used in traditional ethnobotanical medicine. However, little is known about the immunomodulatory activity of essential oils isolated from these plants. Thus, we isolated essential oils from the flowers and leaves of R. albiflorum (cascade azalea) and analyzed their chemical composition and innate immunomodulatory activity. Compositional analysis of flower (REOFl) versus leaf (REOLv) essential oils revealed significant differences. REOFl was comprised mainly of monoterpenes (92%), whereas sesquiterpenes were found in relatively low amounts. In contrast, REOLv was primarily composed of sesquiterpenes (90.9%), with a small number of monoterpenes. REOLv and its primary sesquiterpenes (viridiflorol, spathulenol, curzerene, and germacrone) induced intracellular Ca2+ mobilization in human neutrophils, C20 microglial cells, and HL60 cells transfected with N-formyl peptide receptor 1 (FPR1) or FPR2. On the other hand, pretreatment with these essential oils or component compounds inhibited agonist-induced Ca2+ mobilization and chemotaxis in human neutrophils and agonist-induced Ca2+ mobilization in microglial cells and FPR-transfected HL60 cells, indicating that the direct effect of these compounds on [Ca2+]i desensitized the cells to subsequent agonist activation. Reverse pharmacophore mapping suggested several potential kinase targets for these compounds; however, these targets were not supported by kinase binding assays. Our results provide a cellular and molecular basis to explain at least part of the beneficial immunotherapeutic properties of the R. albiflorum essential oils and suggest that essential oils from leaves of this plant may be effective in modulating some innate immune responses, possibly by inhibition of neutrophil migration.
Keywords: Rhododendron albiflorum, essential oil, calcium flux, neutrophil, chemotaxis, sesquiterpene, microglial cells
1. Introduction
The genus Rhododendron belongs to the Ericaceae family of plants and includes more than 1000 identified species [1]. This genus is a source of flavonoids, tannins, essential oils, chromones, terpenoids, and steroids [2,3]. Rhododendron extracts have been reported to exhibit a diverse range of bioactivities, including antimicrobial, antioxidant, anticancer, antidiabetic, and anti-inflammatory activity [1,4,5,6,7,8,9,10]. Additionally, extracts from various Rhododendron species have been utilized in traditional medicine for their anti-inflammatory properties. For example, R. albiflorum (cascade azalea) has been used in a poultice by the Syilx (Okanagan) and Thompson First Nations people to treat inflammatory conditions and by the Skokomish Indian Tribe as an extract for treating colds, sore throats, and cuts [11]. On the other hand, there are no publications regarding the biological activity of extracts from this plant.
Essential oils represent the volatile fraction of aromatic plants and have been actively investigated for their use in complementary or alternative medicine [12,13,14,15]. Thus, analysis of the chemical composition of essential oils from different plant species, and further evaluation of their biological properties, including immunomodulatory activity, can lead to the discovery of novel therapeutics. For example, essential oils from some Rhododendron species have been reported to be comprised of monoterpenes, sesquiterpenes, and their oxygenated derivatives (Table 1). These essential oils have also been found to be pharmacologically active [16], although little is known about their immunomodulatory activity and effects on innate immune system function.
Table 1.
Review of the major volatile constituents of Rhododendron essential oils.
| Species | Major Compounds (%) | Ref. |
|---|---|---|
| R. tomentosum | Sabinene (0–33), myrcene (0–55.7), p-cymene (0–51.7), limonene (0–50.3), γ-terpineol (0–31.2), bornyl acetate (0–10.8), ascaridol isomers (0–49.2), palustrol (0–53.5), ledol (0–36.5), lepalol (3.3–7.9), lepalone (0.7–6.5), and cyclocolorenone isomers (4.1) | [17,18,19] |
| R. anthopogonoides | 4-Phenyl-2-butanone (27.2), nerolidol (8.1), 1,4-cineole (7.9), caryophyllene (7.6), γ-elemene (6.1), α-farnesene (4.4), and spathulenol (4.2) | [20] |
| R. capitatum | Cedrene (22.2), 1,4,7,-cycloundecatriene,1,5,9,9-tetramethyl-,Z,Z,Z (18.5), α-gurjunene (5.1), α-selinene (4.8), and eremophilene (7.7) | [21] |
| R. przewalskii | Bisabolol oxide II (10.4), 4-(2,3,4,6-tetramethylphenyl)-3-buten-2-one (27.7), and manoyl oxide (10.8) | [21] |
| R. mucronulatum | Borneol (36.6), β-caryophyllene, α-humulene (15.4), and germacrene D (5.3) | [21] |
|
R. micranthum
R. micranthum |
Germacrene D (27.6), α-humulene (6.1), α-muurolene (4.6), δ-cadinene, spathulenol (5.1), 15-copaenol (5.4), α-cadinol (6.3), and τ-muurolol (6.1) | [21] |
| R. anthopogon | α-Pinene (21.5–37.4), δ-cadinene (9.1–13.8), β-pinene (9.5–16.0), limonene (5.9–13.3), cis-ocimene (5.3), δ-amorphene (4.6), α-muurolene (4.5), and (E)-caryophyllene (3.2) | [22,23] |
The innate immune system is essential for host defense against infection. Among the earliest cell types responding to the presence of pathogenic organisms are macrophages and neutrophils [24]. Neutrophils are especially essential for early innate immune response and perform a variety of complex microbicidal functions, including phagocytosis, chemotaxis, and destruction of pathogens [25]. Thus, neutrophils represent an ideal pharmacological target for therapeutic development [26,27,28]. Indeed, several natural products have been evaluated for their neutrophil immunomodulatory activity [29,30,31,32].
The innate immune cells associated with most chronic neurodegenerative diseases are microglial cells [33]. These cells are resident macrophages of the central nervous system (CNS); phagocytose cellular debris; and foreign antigens, and contribute to pathological events, such as inflammation [34]. Microglial cells are capable of upregulating the synthesis and release of various inflammatory mediators [35], and excessive microglial activation can induce inflammation-mediated neuronal damage and degeneration. Numerous herbal compounds have been reported to suppress neurotoxicity via inhibiting microglial activation [36], and some essential oils or component compounds have been shown to have anti-inflammatory activity in microglial cells. For example, essential oils isolated from Artemisia herba-alba and Schisandra chinensis were reported to inhibit nitric oxide (NO) production induced by lipopolysaccharide (LPS) in murine BV2 microglial cells [37,38]. Similarly, linalool was reported to inhibit LPS-induced tumor necrosis factor (TNF), interleukin-1β, and NO production by BV2 cells [39]. On the other hand, the effects of R. albiflorum essential oils on microglial cells has not been evaluated.
Based on the reported anti-inflammatory properties of Rhododendron extracts, we hypothesized that some components in these extracts could have immunomodulatory activity. Additionally, the wide range of studies demonstrating immunomodulatory activity of essential oils led to the hypothesis that Rhododendron essential oils could be contributing to these therapeutic properties. Thus, we evaluate the chemical composition and immunomodulatory activity of essential oils isolated from the flowers and leaves of R. albiflorum. We show that essential oils from the leaves of R. albiflorum had a high content of sesquiterpenes, including viridiflorol, curzerene, spathulenol, bicyclogermacrene, germacrene B, and germacrone. Furthermore, we show that essential oils isolated from R. albiflorum leaves but not flowers inhibited neutrophil and microglial functional responses, including intracellular Ca2+ mobilization and chemotaxis. Likewise, four of the major individual sesquiterpenes identified in R. albiflorum leaf essential oils also inhibited these functional responses, further defining the active components. Given the critical role of neutrophils and microglial cells in inflammation, our data support the possibility that these sesquiterpenes could be effective therapeutic compounds for the development anti-inflammatory agents.
2. Materials and Methods
2.1. Plant Material
R. albiflorum is found in British Columbia, Washington, Oregon, and western Montana. For these studies, we collected R. albiflorum flowers and leaves in July of 2020 during the flowering and fruiting stages on the west side of Table Mountain, Gallatin County, Montana, USA at an elevation of 2820 m above sea level. Botanical identification of the plant material was performed by botanist Robyn A. Klein from Montana State University (Bozeman, MT, USA). The samples were air-dried for 7–10 days at room temperature away from direct sunlight.
2.2. Materials
Dimethyl sulfoxide (DMSO), N-formyl-Met-Leu-Phe (fMLF), phorbol 12-myristate 13-acetate (PMA), Trp-Lys-Tyr-Met-Val-Met (WKYMVM), Histopaque 1077, and viridiflorol were purchased from Sigma-Aldrich Chemical Co. (St. Louis, MO, USA). Germacrone and β-phellandrene were purchased from TargetMol (Boston, MA, USA). Curzerene was purchased from ChemNorm (Wuhan, China), and spathulenol was purchased from ChemFaces (Wuhan, China). n-Hexane was purchased from Merck (Darmstadt, Germany). Fluo-4AM was purchased from Invitrogen (Carlsbad, CA, USA), and FLIPR Calcium 5 was from Molecular Devices (Sunnyvale, CA, USA). Roswell Park Memorial Institute (RPMI) 1640 medium and Dulbecco’s Modified Eagle’s Medium (DMEM):F12 medium were purchased from HyClone Laboratories (Logan, UT, USA). Fetal calf serum and fetal bovine serum (FBS) were purchased from ATCC (Manassas, VA, USA). Hanks’ balanced salt solution was purchased from Life Technologies (Grand Island, NY, USA). HBSS without Ca2+ and Mg2+ is designated as HBSS–; HBSS containing 1.3 mM CaCl2 and 1.0 mM MgSO4 is designated as HBSS+.
2.3. Essential Oil Isolation
Essential oils were obtained by hydrodistillation of dried plant material using a Clevenger type apparatus [31]. The yield of the essential oil was calculated based on the amount of air-dried plant material used. Stock solutions of the essential oils were prepared in DMSO (10 mg/mL) for biological evaluation and in n-hexane (10% w/v) for gas-chromatographic (GC) analysis.
2.4. Gas Chromatography-Mass Spectrometry (GC-MS) Analysis
GC-MS analysis was performed with an Agilent 5975 GC-MSD system (Agilent Technologies, Santa Clara, CA, USA) [40]. An Agilent Innowax FSC column (60 m × 0.25 mm, 0.25 μm film thickness) was used with He as the carrier gas (0.8 mL/min). The GC oven temperature was kept at 60 °C for 10 min, increased to 220 °C at a rate of 4 °C/min, kept constant at 220 °C for 10 min, and then increased to 240 °C at a rate of 1 °C/min. Samples of 1 µL were injected, and the split ratio was adjusted to 40:1 to prevent overloading of the detectors. The injector temperature was 250 °C. MS spectra were monitored at 70 eV with a mass range of 35 to 450 m/z. GC analysis was carried out using an Agilent 6890N GC system. To obtain the same elution order as with GC-MS, the line was split for the flame ionization (FI) and MS detectors, and a single injection was performed using the same column and appropriate operational conditions. The FI detector (FID) temperature was 300 °C. The essential oil components were identified by co-injection with standards (whenever possible), which were purchased from commercial sources or isolated from natural sources. In addition, compound identities were confirmed by comparison of their mass spectra with those in the Wiley GC-MS Library (Wiley, NY, USA), MassFinder software 4.0 (Dr. Hochmuth Scientific Consulting, Hamburg, Germany), Adams Library, and NIST Library. Confirmation was also achieved using the in-house “Başer Library of Essential Oil Constituents” database, obtained from chromatographic runs of pure compounds performed with the same equipment and conditions. A C8–C40 n-alkane standard solution (Fluka, Buchs, Switzerland) was used to spike the samples for the determination of relative retention indices (RRI). Relative percentage amounts of the separated compounds were calculated from FID chromatograms.
2.5. Isolation of Human Neutrophils
For isolation of human neutrophils, blood was collected from healthy donors in accordance with a protocol approved by the Institutional Review Board at Montana State University (Protocol #MQ041017), as described previously [27]. Isolated neutrophils were washed and resuspended in HBSS–. Neutrophil preparations were routinely >95% pure and >98% viable. Neutrophils were obtained from multiple different donors (n = 8); however, the cells from different donors were never pooled during experiments.
2.6. Cell Culture
Human promyelocytic leukemia HL60 cells stably transfected with FPR1 (FPR1-HL60 cells) or FPR2 (FPR2-HL60 cells) were kindly provided by Dr. Marie-Josephe Rabiet, INSERM, Grenoble, France. These cells were cultured in RPMI 1640 medium containing 10% heat-inactivated fetal calf serum, 10 mM HEPES, 100 μg/mL streptomycin, 100 U/mL penicillin, and G418 (1 mg/mL). G418 was removed before assays were performed.
Human C20 microglial cells were kindly provided by Dr. David Alvarez-Carbonell (Department of Molecular Biology and Microbiology, Case Western Reserve University, Cleveland, OH, USA); these are immortalized primary human microglial cells that maintain characteristics of primary microglial cells [41]. C20 cells were grown at 37 °C and 5% CO2 in DMEM:F12 medium supplemented with 10% FBS, 2.5 mM L-glutamine, 110 mg/L sodium pyruvate, 15 mM HEPES, 100 μg/mL normocin, 100 μg/mL streptomycin, and 100 U/mL penicillin.
2.7. Ca2+ Mobilization Assay
Changes in intracellular Ca2+ concentrations ([Ca2+]i) in human neutrophils were measured with a FlexStation 3 scanning fluorometer (Molecular Devices), as previously described [27]. To assess the direct effects of test compounds or pure essential oils on Ca2+ influx, the compounds/oils were added to the wells (final concentration of DMSO was 1%), and changes in fluorescence were monitored (λex = 485 nm, λem = 538 nm) every 5 s for 240 s at room temperature after addition of the test compound. To evaluate inhibitory effects of the compounds on FPR1/FPR2-dependent Ca2+ influx, the compounds were added to the wells (final concentration of DMSO was 1%) with cells (human neutrophils or FPR1/FPR2 HL60 cells). The samples were preincubated for 10 min, followed by addition of 5 nM fMLF (for human neutrophils or FPR1-HL60 cells) or 5 nM WKYMVM (for FPR2-HL60 cells). The maximum change in fluorescence, expressed in arbitrary units over baseline, was used to determine the agonist response. Responses were normalized to the response induced by 5 nM fMLF or 5 nM WKYMVM, which were assigned as 100%.
For analysis of Ca2+ influx in C20 microglial cells, the cells were plated in 96-well black, clear bottom plates at 104 cells/well in DMEM/F12 medium containing 10% FBS. The cells were treated with 200 nM PMA for 24 h, and the medium was changed every day for 5 days. On day 5, the cells were loaded with FLIPR Calcium 5 (Molecular Devices) at a volume ratio of 1:1 for 30 min at 37 °C in the dark. The plates were then placed in a FlexStation 3 fluorometer, and basal fluorescence was measured (λex = 485 nm, λem = 538 nm). Essential oils or individual compounds of interest were added manually (final concentration of DMSO was 1%), and fluorescence was monitored for 2 min to assess the direct effects of these treatments on [Ca2+]i. After a 10 min incubation at 37 °C, the fluorescence baseline was recorded again, and 10 µM fMLF was added to evaluate inhibitory effects on agonist-induced [Ca2+]i. Responses were normalized to the response induced by 10 µM fMLF.
For all Ca2+ influx experiments, curve fitting (at least five or six points) and calculation of median effective concentration values (EC50 or IC50) were performed by nonlinear regression analysis of the dose–response curves generated using Prism 7 (GraphPad Software, Inc., San Diego, CA, USA).
2.8. Chemotaxis Assay
Human neutrophils were resuspended in HBSS+ containing 2% (v/v) heat-inactivated fetal bovine serum (2 × 106 cells/mL), and chemotaxis was analyzed in 96-well ChemoTx chemotaxis chambers (Neuroprobe, Gaithersburg, MD), as described previously [27]. Curve fitting (at least eight to nine points) and calculation of median effective concentration values (IC50) were performed by nonlinear regression analysis of the dose-response curves generated using GraphPad Prism 8.
2.9. Cytotoxicity Assay
Cytotoxicity of essential oils and pure compounds was analyzed in human promyelocytic HL60 cells using a CellTiter-Glo Luminescent Cell Viability Assay Kit (Promega), as described previously [31].
2.10. Kinase Kd Determination
KINOMEscan®® was used to determine the dissociation constant (Kd) of the indicated sesquiterpenes for selected kinases [42] (Eurofins Pharma Discovery, San Diego, CA, USA). A 12-point half-log dilution series (maximum concentration of 33 µM) was used for Kd determination. Assays were performed in duplicate, and the average mean value is shown.
2.11. Molecular Modeling
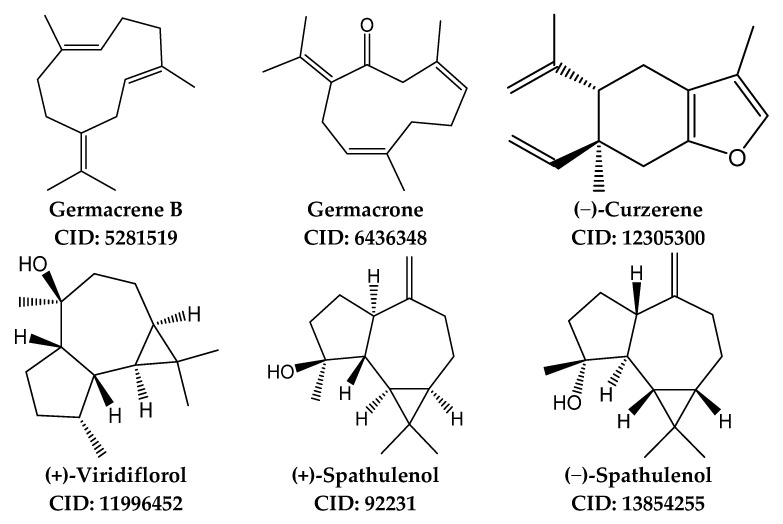
PharmMapper [43] was used for identifying putative protein targets for (−)-curzerene, (+) and (−) enantiomers of spathulenol, germacrene B, germacrone, and (+)-viridiflorol. For a given small molecule, PharmMapper recognizes potential target possibilities using an “invert” pharmacophore mapping methodology. In several reference databases that are incorporated in the software, the protein biotargets are represented by sets of pharmacophore points that provide faster mapping. The PubChem database (https://pubchem.ncbi.nlm.nih.gov; accessed 20 February2021) was used as a source of initial 3D structures for the investigated compounds. The structures of (−)-curzerene (CID: 12305300), (−)-spathulenol (CID: 13854255), (+)-spathulenol (CID: 92231), germacrene B (CID: 5281519), germacrone (CID: 6436348), and (+)-viridiflorol (CID: 11996452) were downloaded from PubChem in SDF format and further uploaded into PharmMapper. Up to 300 conformers of each compound were automatically generated using a corresponding option of the software. Pharmacophore mapping was performed with the “Human Protein Targets Only” database containing 2241 targets. The top 250 potential targets per compound were retrieved and sorted by the normalized fit score. The physicochemical properties of selected compounds were computed using SwissADME (http://www.swissadme.ch; accessed 25 February 2021) [44]. Structures of the main sesquiterpenes found in REOLv and used for molecular modeling are shown in Figure 1.
Figure 1.
Chemical structures of major sesquiterpenes found in essential oils isolated from the leaves of R. albiflorum.
2.12. Statistical Analysis
One-way analysis of variance (ANOVA) was performed on the data sets, followed by Tukey’s pair-wise comparisons. Pair-wise comparisons with differences at p < 0.05 were considered to be statistically significant.
3. Results and Discussion
3.1. Essential Oil Composition
The yields (v/w) of essential oils obtained from R. albiflorum flowers (designated as REOFl) and leaves (designated as REOLv) were 0.4% and 0.5%, respectively. The chemical composition of these essential oils was evaluated using simultaneous GC-FID and GC-MS, and Table 2 summarizes the identified compounds, percentage composition, and relative retention indices (RRI) (compounds are listed in order of their elution).
Table 2.
Chemical composition of R. albiflorum essential oils (%) isolated from flowers (REOFl) and leaves (REOLv) a.
| No | RRI | Compound | REOLv | REOFl | N° | RRI | Compound | REOLv | REOFl |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1032 | α-Pinene | t | 1.2 | 33 | 1612 | β-Caryophyllene | 0.4 | |
| 2 | 1076 | Camphene | t | 0.1 | 34 | 1650 | γ-Elemene | 2.4 | 0.6 |
| 3 | 1118 | β-Pinene | t | 1.1 | 35 | 1661 | Alloaromadendrene | t | |
| 4 | 1132 | Sabinene | 0.9 | 36 | 1662 | Pulegone | t | ||
| 5 | 1174 | Myrcene | 1.2 | 37 | 1668 | (Z)-β-Farnesene | t | ||
| 6 | 1176 | α-Phellandrene | 0.6 | 38 | 1687 | α-Humulene | 0.9 | ||
| 7 | 1188 | α-Terpinene | 0.4 | 39 | 1704 | γ-Curcumene | 0.4 | ||
| 8 | 1203 | Limonene | t | 14.2 | 40 | 1719 | Borneol | 1.1 | |
| 9 | 1218 | β-Phellandrene | 8.9 | 41 | 1726 | Germacrene D | 0.5 | ||
| 10 | 1246 | (Z)-β-Ocimene | 6.5 | 42 | 1742 | β-Selinene | t | ||
| 11 | 1255 | γ-Terpinene | 7.1 | 43 | 1744 | α-Selinene | t | ||
| 12 | 1266 | (E)-β-Ocimene | 3.4 | 44 | 1755 | Bicyclogermacrene | 8.9 | 0.4 | |
| 13 | 1280 | p-Cymene | 2.8 | 45 | 1786 | ar-Curcumene | 0.6 | ||
| 14 | 1290 | Terpinolene | t | 37.7 | 46 | 1815 | 2-Tridecanone | 0.5 | |
| 15 | 1382 | cis-Alloocimene | 0.5 | 47 | 1854 | Germacrene B | 6.8 | ||
| 16 | 1398 | 2-Nonanone | t | 48 | 1886 | Curzerene | 17.8 | 2.2 | |
| 17 | 1437 | α-Thujone | t | 49 | 2050 | (E)-Nerolidol | 0.2 | ||
| 18 | 1443 | 2,5-Dimethylstyrene | 0.4 | 50 | 2096 | Elemol | 1.4 | ||
| 19 | 1451 | β-Thujone | 1.2 | 51 | 2104 | Viridiflorol | 22.0 | 1.2 | |
| 20 | 1477 | 4,8-Epoxyterpinolene | 0.9 | 52 | 2106 | β-Elemenone | 5.3 | 1.7 | |
| 21 | 1479 | δ-Elemene | t | 53 | 2144 | Spathulenol | 14.4 | 0.3 | |
| 22 | 1495 | Bicycloelemene | t | 54 | 2147 | Germacrone | 3.3 | ||
| 23 | 1528 | α-Bourbonene | t | 55 | 2198 | Thymol | 0.3 | ||
| 24 | 1535 | β-Bourbonene | t | 56 | 2199 | Alismol | 1.3 | ||
| 25 | 1536 | Italicene | t | 57 | 2203 | β-Eudesmol | 0.3 | ||
| 26 | 1541 | Benzaldehyde | t | 58 | 2219 | Porosadienol | 0.6 | ||
| 27 | 1545 | cis-α-Bergamotene | t | 59 | 2217 | Alismol isomer | 1.2 | ||
| 28 | 1553 | Linalool | 0.5 | 60 | 2368 | Eudesma-4(15),7-diene-1-β-ol | t | ||
| 29 | 1590 | Bornyl acetate | 0.6 | 0.2 | |||||
| 30 | 1600 | β-Elemene | 2.2 | 61 | 2400 | Tetracosane | 0.5 | ||
| 31 | 1604 | 2-Undecanone | 0.2 | 62 | 2500 | Pentacosane | 0.9 | ||
| 32 | 1611 | Terpinen-4-ol | 0.8 | 63 | 2656 | Furanoeremophil-1-one | t |
a The data are presented as relative % for each component that was identified in REOFl and REOLv. RRI, relative retention index calculated on the basis of retention of n-alkanes; %, calculated from flame ionization detector data. Trace amounts (t) were present at <0.1%. All other compounds were identified by comparison with co-injected standards. Major component compounds (>2%) are indicated in bold.
A total of 63 constituent compounds were identified in R. albiflorum essential oils. Specifically, 34 compounds were identified in REOFl, representing ~99.8% of the total essential oil composition. The main components of REOFl were terpinolene (37.7%), limonene (14.2%), β-phellandrene (8.9%), γ-terpinene (7.1%), (Z)-β-ocimene (6.5%), p-cymene (2.8%), and curzerene (2.2%). Twenty-three other compounds were present at concentrations from 0.1% to <2.0%. In comparison, 41 compounds were identified in REOLv, representing ~92.2% of the total essential oil composition. The main components of REOLv were viridiflorol (22.0%), curzerene (17.8%), spathulenol (14.4%), bicyclogermacrene (8.9%), germacrene B (6.8%), β-elemenone (5.3%), germacrone (3.3%), γ-elemene (2.4%), and β-elemene (2.2%). Fourteen other compounds were present at concentrations from 0.1% to <2%. The remaining volatile compounds identified in both essential oil samples were present in trace amounts (<0.1%). Overall, there were significant differences in essential oil composition between R. albiflorum flowers and leaves, with the major components of REOFl being monoterpenes, such as monoterpene hydrocarbons (87%) and oxygenated monoterpenes (5.0%). In contrast, the main components of REOLv were sesquiterpene hydrocarbons (40.9%) and oxygenated sesquiterpenes (50.0%) (Table 3). Note that spathulenol, which we found to be present in REOLv, was previously reported to be a major component compound in R. micranthum essential oils [21]. However, this is the first report to show that the sesquiterpenoids viridiflorol, curzerene, bicyclogermacrene, germacrene B, and germacrone are also major components of essential oils from Rhododendron spp.
Table 3.
Summary of the chemical compositions of R. albiflorum essential oils.
| Major Components | REOLv | REOFl |
|---|---|---|
| % | ||
| Monoterpene hydrocarbons | <0.1 | 87.0 |
| Oxygenated monoterpenes | 0.6 | 5.0 |
| Sesquiterpene hydrocarbons | 40.9 | 3.2 |
| Oxygenated sesquiterpenes | 50.0 | 3.2 |
| Miscellaneous compounds | 0.7 | 1.4 |
| Total | 92.2 | 99.8 |
3.2. Effect of the R. albiflorum Essential Oils and Component Compounds on Neutrophil and Microglial [Ca2+]i
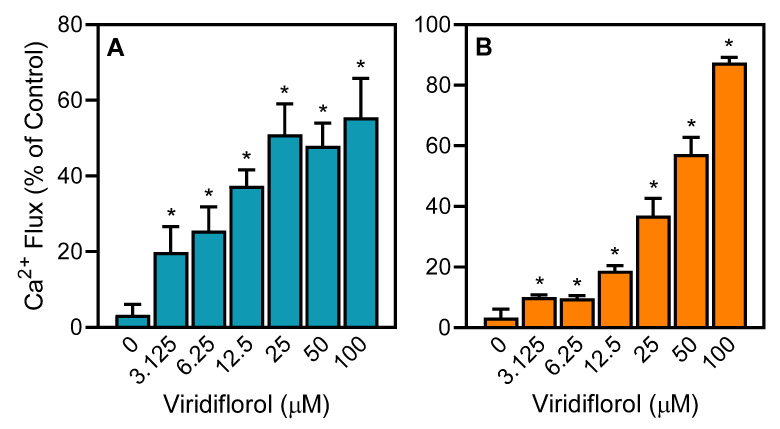
R. albiflorum essential oils and commercially available individual compounds were evaluated for their effects on human neutrophils and human C20 microglial cells. Specifically, we evaluated their effects on [Ca2+]i, which is a key component of phagocyte activation [45,46]. We found that REOLv treatment increased [Ca2+]i, with EC50 values of 18.6 µg/mL and 22.8 µg/mL in neutrophils and C20 microglial cells, respectively (Table 4). In addition, analysis of the major sesquiterpenes that comprised 57.5% of REOLv (viridiflorol, spathulenol, curzerene, and germacrone) showed that these compounds also activated neutrophil Ca2+ influx, with the most potent being viridiflorol (Table 4, Figure 2). Likewise, viridiflorol, curzerene, and germacrone also increased C20 microglial cell [Ca2+]i, whereas spathulenol was inactive or had very low activity in these cells (Table 4). In any case, it is clear that viridiflorol, which is the major compound in REOLv, is one of the principal molecules responsible for neutrophil and microglial cell activation.
Table 4.
Effect of R. albiflorum essential oils and component compounds on Ca2+ influx in human neutrophils and microglial cells.
| Essential Oil or Pure Compound | Ca2+ Influx | |||
|---|---|---|---|---|
| Neutrophils | C20 cells | |||
| EC50 (µg/mL) | ||||
| REOLv | 18.6 ± 5.8 | 22.8 ± 1.6 | ||
| REOFl | N.A. | N.A. | ||
| REOLv | REOFl | EC50 (µM) | ||
| Composition (%) | ||||
| β-Phellandrene | 0 | 8.9 | N.A. | N.A. |
| Viridiflorol | 22.0 | 1.2 | 6.8 ± 2.3 | 27.8 ± 4.6 |
| Spathulenol | 14.4 | 0.3 | 39.4 ± 9.5 | N.A. |
| Curzerene | 17.8 | 2.2 | 37.6 ± 8.4 | 25.9 ± 5.2 |
| Germacrone | 3.3 | 0 | 24.0 ± 4.6 | 27.7 ± 2.5 |
EC50 values were determined by nonlinear regression analysis of the dose-response curves as described under Materials and Methods. N.A. indicates the samples had essentially no activity (EC50 >50 µM or >50 µg/mL). The data are presented as the mean ± SD of three independent experiments.
Figure 2.
Effect of viridiflorol on neutrophil and microglial Ca2+ mobilization. Human neutrophils (A) and human C20 microglial cells (B) were treated with the indicated concentrations of viridiflorol, and [Ca2+]i was measured as described. The data are expressed as the change in [Ca2+]i and compared to control [Ca2+]i induced by 5 nM fMLF (100%) in neutrophils or 10 μM fMLF (100%) in microglial cells and plotted as mean ± SD. The data presented are from one experiment that is representative of two independent experiments with similar results. * p < 0.01 compared to DMSO control [Ca2+]i.
In contrast to REOLv, REOFl had no effect on neutrophil or C20 microglial cell [Ca2+]i (Table 4). Similarly, β-phellandrene, which comprises 8.9% of REOFl, was also inactive in both of these cell types (Table 4). Previously, we analyzed a number of monoterpenes, including 12 compounds that we found here to comprise 77.8% of REOFl, for their ability to activate neutrophil Ca2+ influx. These compounds, including α-pinene, β-pinene, sabinene, myrcene, α-terpinene, limonene, (E/Z)-β-ocimene, γ-terpinene, p-cymene, terpinolene, linalool, and terpinen-4-ol, were all found previously to have no activating effects on neutrophil Ca2+ mobilization [29,31]. Thus, these previous studies together with our current results help to explain why REOFl is also inactive.
3.3. Effect of R. albiflorum Essential Oils and Component Compounds on Agonist-Induced Ca2+ Influx
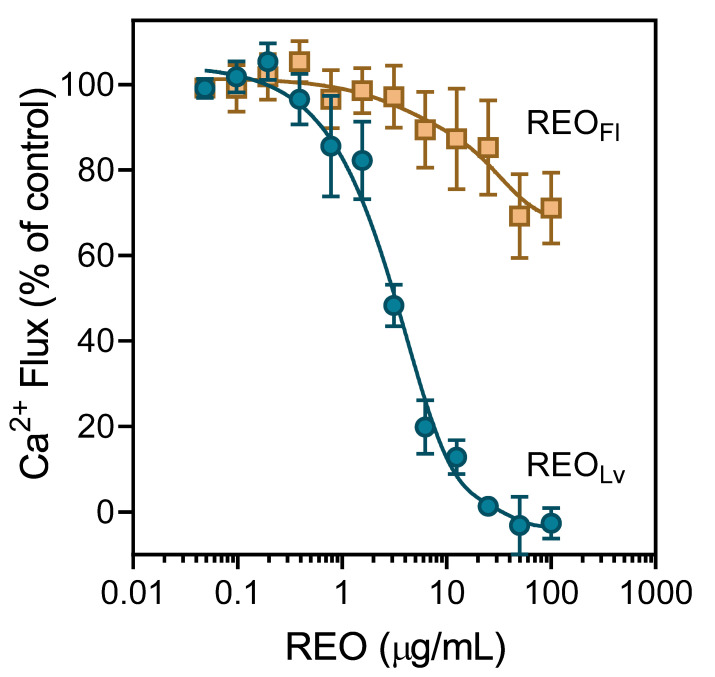
Activation of Ca2+ influx, specific receptors, or other unidentified molecular targets by agonists can result in the desensitization and subsequent downregulation of neutrophil responses [29,47]. Essential oils and their components have been reported previously to modulate [Ca2+]i and inhibit cell migration [29,30,31,32]. Thus, we evaluated R. albiflorum essential oils for their effects on agonist-induced neutrophil and microglial cell activation. As shown in Figure 3, REOLv potently inhibited neutrophil Ca2+ influx induced by the agonist fMLF, with an IC50 of 2.7 μg/mL. Similarly, REOLv inhibited Ca2+ influx in fMLF-activated C20 microglial cells and FPR1-HL60 cells, as well as in WKYMVM-activated FPR2-HL60 cells (Table 5). As expected from our results above, REOFl had little effect on [Ca2+]i in fMLF-stimulated neutrophils, even at very high REOFl concentrations (Figure 3). Similarly, REOFl had no effect on [Ca2+]i in microglial, FPR1-HL60, and FPR2-HL60 cells (Table 5).
Figure 3.
Effect of R. albiflorum essential oils on fMLF-induced neutrophil Ca2+ mobilization. Human neutrophils were treated with the indicated concentrations of the REOLv, REOFl, or 1% DMSO (negative control) for 10 min. The cells were then activated by 5 nM fMLF, and [Ca2+]i was monitored as described. The data shown are presented as the mean ± SD from one experiment that is representative of three independent experiments with similar results.
Table 5.
Effect of R. albiflorum essential oils and component compounds on agonist-induced functional responses in human neutrophils and microglial cells.
| Essential Oil or Pure Compound | FPR1- HL60 a | FPR2- HL60 b | C20 Cells a | Neutro-phils a | Neutrophils c | ||
|---|---|---|---|---|---|---|---|
| Ca2+ Influx | Chemotaxis | ||||||
| IC50 (µg/mL) | |||||||
| REOLv | 12.3 ± 2.5 | 7.6 ± 2.3 | 8.0 ± 0.1 | 2.7 ± 0.6 | 3.3 ± 0.5 | ||
| REOFl | N.A. | N.A. | N.A. | N.A. | N.A. | ||
| Composition (%) | |||||||
| REOLv | REOFl | IC50 (µM) | |||||
| β-Phellandrene | 0 | 8.9 | N.A. | N.A. | N.A. | N.A. | N.A. |
| Viridiflorol | 22.0 | 1.2 | 19.5 ± 4.7 | 10.7 ± 3.8 | 22.6 ± 3.1 | 7.8 ± 2.3 | 18.3 ± 4.1 |
| Spathulenol | 14.4 | 0.3 | 32.2 ± 6.4 | 31.6 ± 5.3 | 9.8 ± 3.4 | 36.2 ± 8.2 | 4.9 ± 0.8 |
| Curzerene | 17.8 | 2.2 | 21.8 ± 6.1 | 16.7 ± 5.5 | 30.7 ± 4.4 | 11.0 ± 3.8 | 37.9 ± 2.2 |
| Germacrone | 3.3 | 0 | 27.7 ± 2.9 | 25.0 ± 7.2 | 10.7 ± 2.3 | 27.9 ± 8.9 | 8.5 ± 0.6 |
a Ca2+ influx was induced by 5 nM fMLF in HL60-FPR1 cells and primary human neutrophils or 10 μM fMLF in human C20 microglial cells. b Ca2+ influx was induced by 5 nM WKYMVM in HL60-FPR2 cells. c Neutrophil chemotaxis was induced by 1 nM fMLF. N.A. indicates the samples had essentially no activity (IC50 > 50 µM or > 50 µg/mL). The data are presented as the mean ± SD of three independent experiments.
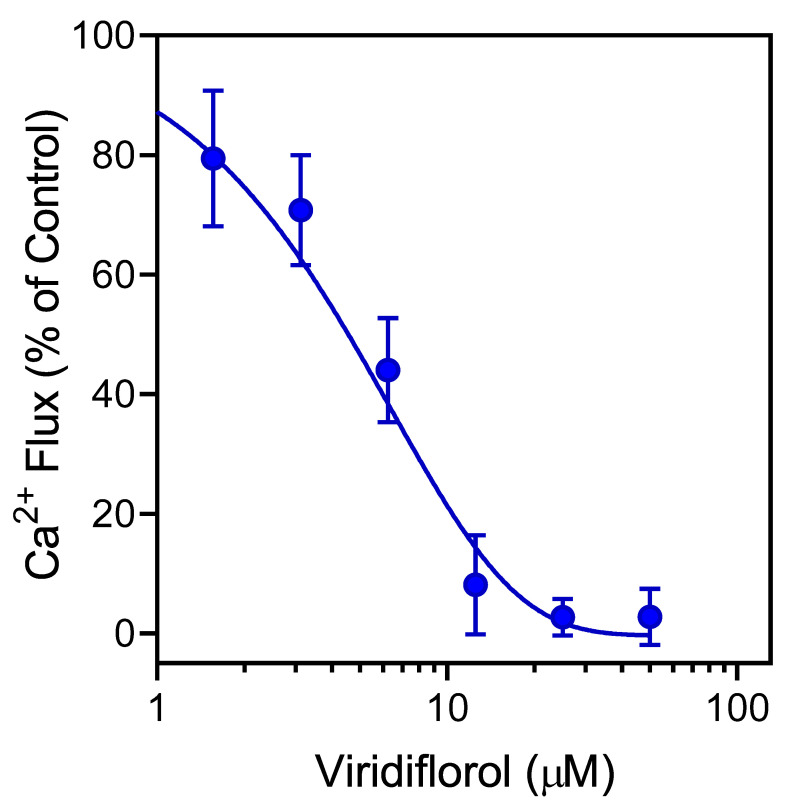
We next evaluated the effects of individual constituent compounds on agonist-induced Ca2+ mobilization in human neutrophils, C20 microglial cells, and FPR-transfected HL60 cells. As shown in Table 5, the four main sesquiterpenes in REOLv (viridiflorol, curzerene, spathulenol, and germacrone) inhibited fMLF-induced Ca2+ influx in human neutrophils, microglial cells, and FPR1-HL60 cells and in WKYMVM-stimulated FPR2-HL60 cells, with IC50 values in the micromolar range. As an example, the dose-dependent inhibition of fMLF-induced neutrophil Ca2+ mobilization by viridiflorol is shown in Figure 4. These results suggest that the direct effect of these compounds on [Ca2+]i (see Table 4) desensitized the cells to subsequent agonist activation. In support of this idea, we found previously that three other sesquiterpenes that are present in REOLv (β-caryophyllene, α-humulene, and germacrene D) also inhibited agonist-induced Ca2+ mobilization and thus desensitized human neutrophils to further agonist activation [32].
Figure 4.
Effect of viridiflorol on neutrophil Ca2+ mobilization. Human neutrophils were treated with the indicated concentrations of viridiflorol or 1% DMSO (negative control) for 10 min. The cells were activated by 5 nM fMLF, and Ca2+ influx was monitored as described. The data are from one experiment that is representative of three independent experiments.
Evaluation of the effects of β-phellandrene, one of the principal monoterpenes in REOFl (8.9%), on agonist-induced neutrophil or HL60-FPR1/FPR2 Ca2+ influx showed that it had no effect (Table 5), which is consistent with its lack of activity as a direct neutrophil agonist (see Table 4). In addition, our previous studies on a number of compounds that we determined here to comprise 77.9% of REOFl (α-pinene, camphene, β-pinene, sabinene, myrcene, α-terpinene, limonene, (E/Z)-β-ocimene, γ-terpinene, p-cymene, terpinolene, linalool, and terpinen-4-ol) showed that they had no inhibitory effect on agonist-induced neutrophil Ca2+ mobilization [29,31,32]. Thus, these previous results together with our current analysis of β-phellandrene again serve to explain why REOFl does not desensitize neutrophil agonist-induced activation.
3.4. Effect of R. albiflorum Essential Oils and Component Compounds on Neutrophil Chemotaxis
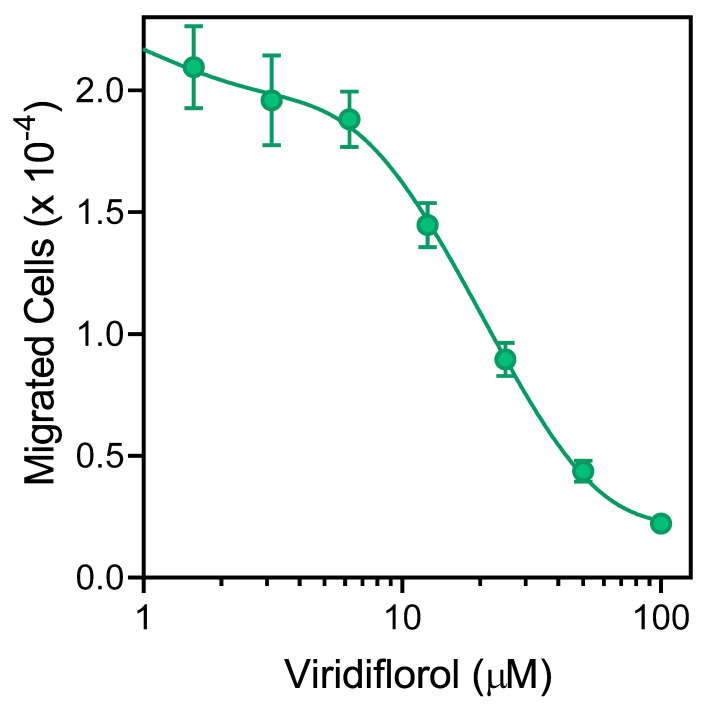
Various essential oils and their components have been reported previously to inhibit cell migration [29,48,49]. We found that pretreatment with REOLv for 10 min dose-dependently inhibited fMLF-induced human neutrophil chemotaxis, with an IC50 of 3.3 µg/mL (Table 5). Likewise, the individual constituent compounds viridiflorol, curzerene, spathulenol, and germacrone also inhibited neutrophil chemotaxis, with the most potent compounds being spathulenol and germacrone (Table 5). As an example, the dose-dependent inhibition neutrophil chemotaxis by viridiflorol is shown in Figure 5. In contrast, REOFl and the monoterpene β-phellandrene were both inactive in this assay (Table 5).
Figure 5.
Inhibition of neutrophil chemotaxis by viridiflorol. Neutrophil migration toward 1 nM fMLF was measured, as described under Materials and Methods. The data are from one experiment that is representative of three independent experiments.
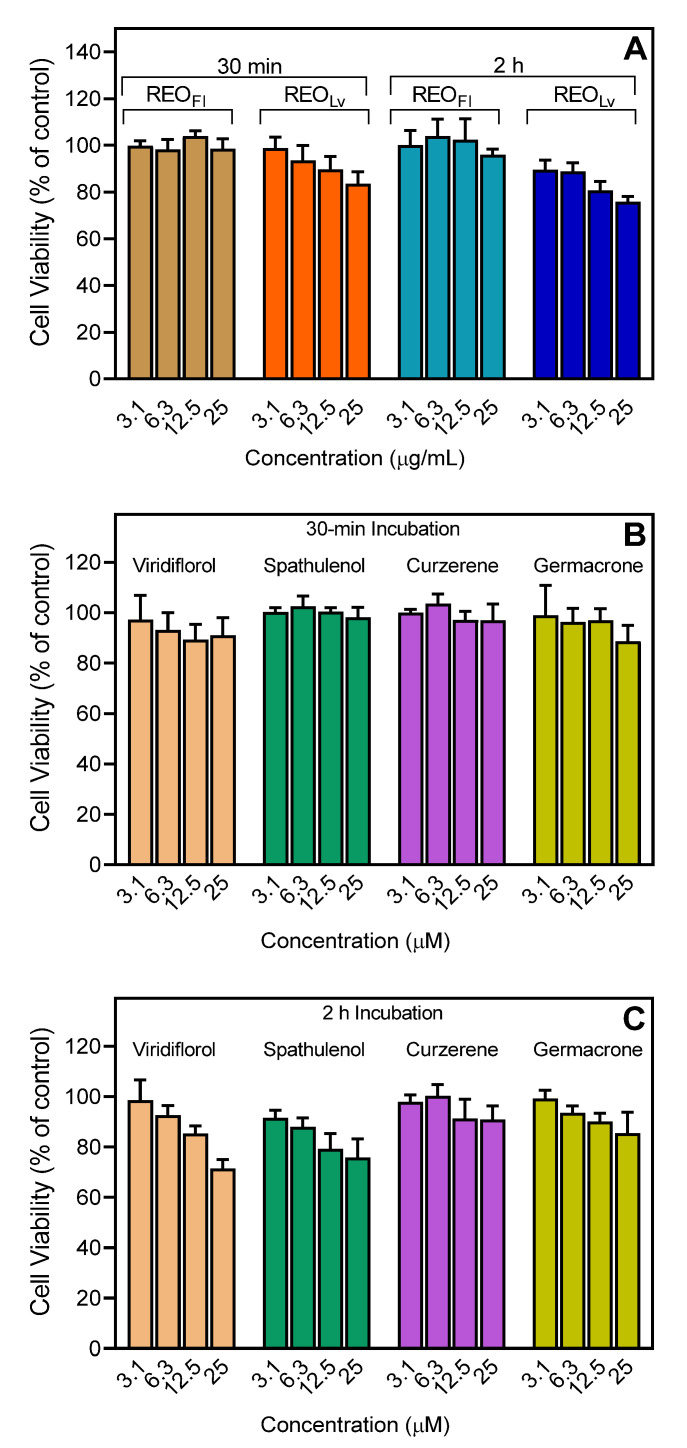
To ensure that the effects of these essential oils or individual compounds on neutrophil functional activity were not influenced by possible toxicity, we evaluated cytotoxicity of REOFl and REOLv (up to 25 µg/mL) and test compounds at various concentrations (up to 25 µM) in HL60 cells during 30 min and 2 h incubation periods. These incubation periods are comparable to the times used to measure Ca2+ mobilization (up to 30 min) and cell migration (up to 1.5 h). As shown in Figure 6, REOLv, REOFl, viridiflorol, spathulenol, curzerene, and germacrone had minimal effects on cell viability during a 30 min incubation, verifying the absence of cytotoxicity during the Ca2+ influx assay period (Figure 6). Likewise, these samples generally had minimal cytotoxicity during the 2 h incubation, except for the highest concentrations of viridiflorol (25 µM and 2-h incubation), which exhibited a little more cytotoxicity that could have some effect on the cell migration assay.
Figure 6.
Cytotoxicity of REOLv, REOFl, and selected sesquiterpenes. HL60 cells were preincubated with REOLv or REOFl (A) or pure compounds (B,C) for 30 min and 2 h, and cell viability was analyzed, as described. Values are the mean ± SD of triplicate samples from one experiment that is representative of two independent experiments with similar results.
3.5. Identification of Potential Protein Targets for Selected Sesquiterpenes
The sesquiterpene compounds identified in REOLv have been reported to exhibit a number of biological activities. For example, viridiflorol has been shown to inhibit carrageenan-induced mouse paw edema [50]. This compound has also been shown to be a potent inhibitor of biofilm formation [51]. Curzerene has been shown to have antiproliferative effects in SPC-A1 human lung adenocarcinoma cells [52] and SKMEL-19 melanoma cells [53]. Spathulenol has been shown to inhibit formalin-induced nociceptive sensitivity and carrageenan-induced mechanical hyperalgesia in mice [54], as well as carrageenan-induced mouse paw oedema [55]. Spathulenol has also been reported to be cytotoxic for B16-F10, HepG2, K562, and HL60 cell lines (IC50 from 18 to 52 µM after 72 h) [56]. Finally, spathulenol has been reported to exhibit spasmolytic acivity, possibly by blocking voltage-operated calcium channels [57]. Germacrone is one of the main bioactive components in the traditional Chinese medicine Rhizoma curcuma [58] and has been reported to possess anti-inflammatory, antiviral, antitumor, and immunomodulatory properties [59,60,61,62,63,64,65,66,67]. For example, germacrone has been reported to alleviate symptoms of collagen-induced arthritis by regulating the T helper type 1 and 2 (Th1/Th2) cell balance and nuclear factor κB (NF-κB) activation [68]. It has also been reported to reduce cerebral ischemia/reperfusion injury in rats via antioxidative and antiapoptotic mechanisms [69]. Finally, germacrone has been reported to inhibit Ca2+-activated Cl− currents and K+ channel activity [70].
Despite the various biological activities reported for these compounds, little is known about their specific cellular targets. Thus, we performed reverse-pharmacophore mapping on the molecular structures of viridiflorol, curzerene, spathulenol, germacrene B, and germacrone to identify potential biological targets. Note that pharmacophore mapping of bicyclogermacrene (comprises 8.9% in REOLv) was previously reported [32]. PharmMapper was used to compare a large database of pharmacophore patterns with these compounds and generate target information, including normalized fitness scores and pharmacophoric characteristics. It is important to submit a compound to the PharmMapper server in the form of the proper optical isomer, as this methodology explicitly accounts for 3D structure of a molecule. Specifically, we evaluated the (+)-configuration of viridiflorol [71] and the (−)-configuration of curzerene, which are the most common enantiomers found in higher plants [72]. Both the (+) and (−) enantiomers of spathulenol have been found in higher plants. For example, (+)-spathulenol was identified in essential oils from Piper species [73], Salvia hydrangea [74], Aloysia gratissima [75], Eremophila mitchellii [76], and extracts from Merremia dissecta [77]. In addition, (−)-spathulenol was found in essential oils from Elytropappus rhinocerotis [78], Annona squamosal [79], Chrysanthemum [80], Artemisia annua [81], and Cleome spinose [82]. Thus, we analyzed both enantiomers of this compound.
The results of PharmMapper analysis (Table 6) indicated that four potential targets were common for five of the investigated compounds: aldo-keto reductase family 1 member C2 (AKR1C2), mitogen-activated protein kinase (MAPK)-activated protein kinase 2 (MAPKAPK2 or MK2), bone morphogenetic protein 2 (BMP2), and c-Jun N-terminal kinase 3 (JNK3). They are present among the eight top-ranked targets found by PharmMapper. Caspase-7 was common for four sesquiterpenes; steroid sulfatase and integrin α-L (CD11a) were common targets for three of the compounds; kinesin-like protein KIF11, proviral integration Moloney virus kinase (PIM1), serum albumin, JNK1, thyroid hormone receptor β (NR1A2), prothrombin, and complement factor B were common targets for two sesquiterpenes; and carbonic anhydrase 2 (CA2) and vascular endothelial growth factor receptor 2 (VEGFR) were potential targets only for (+)-viridiflorol.
Table 6.
Potential protein targets identified by PharmMapper for germacrene B, germacrone, curzerene, (−)-viridiflorol, and (+)- and (−)-spathulenol.
| Rank | PDB ID | Target Name | Fit Score | Rank | PDB ID | Target Name | Fit Score |
|---|---|---|---|---|---|---|---|
| Germacrene B | Germacrone | ||||||
| 1 | 1J96 | AKR1C2 | 0.9912 | 1 | 1J96 | AKR1C2 | 0.9926 |
| 2 | 1REU | BMP2 | 0.9846 | 2 | 1PMV | JNK3 | 0.9911 |
| 3 | 2P3G | MAPKAPK2 | 0.9817 | 3 | 1UKI | JNK1 | 0.9909 |
| 4 | 2PG2 | KIF11 | 0.9735 | 4 | 2PIN | NR1A2 | 0.9712 |
| 5 | 1P49 | Steroid sulfatase | 0.9567 | 5 | 1P49 | Steroid sulfatase | 0.9586 |
| 6 | 1SHJ | Caspase-7 | 0.9481 | 6 | 2PG2 | KIF11 | 0.9537 |
| 7 | 1E7E | Serum albumin | 0.9419 | 7 | 1L6L | Apo A-II | 0.9489 |
| 8 | 2O65 | PIM1 | 0.9295 | 8 | 1RS0 | CFB | 0.9415 |
| (−)-Curzerene | ( + )-Viridiflorol | ||||||
| 1 | 1REU | BMP2 | 0.9904 | 1 | 1XDD | Integrin α-L | 3 |
| 2 | 2PIN | NR1A2 | 0.9873 | 2 | 1J96 | AKR1C2 | 3 |
| 3 | 2O65 | PIM1 | 0.9861 | 3 | 3BMP | BMP2 | 3 |
| 4 | 2P3G | MAPKAPK2 | 0.9764 | 4 | 2P3G | MAPKAPK2 | 2.906 |
| 5 | 1UKI | JNK1 | 0.975 | 5 | 1IF4 | CA2 | 2.886 |
| 6 | 1PMV | JNK3 | 0.9671 | 6 | 3CJF | VEGFR2 | 2.881 |
| 7 | 1RS0 | CFB | 0.9652 | 7 | 1SHJ | Caspase-7 | 2.708 |
| 8 | 1SHJ | Caspase-7 | 0.9594 | 8 | 3CGF | JNK3 | 2.568 |
| (−)-Spathulenol | (+)-Spathulenol | ||||||
| 1 | 1XDD | Integrin α-L | 2.968 | 1 | 2P3G | MAPKAPK2 | 2.952 |
| 2 | 1NO9 | Prothrombin | 2.948 | 2 | 3BMP | BMP2 | 2.947 |
| 3 | 3BMP | BMP2 | 2.947 | 3 | 1XDD | Integrin α-L | 2.895 |
| 4 | 1J96 | AKR1C2 | 2.921 | 4 | 1NO9 | Prothrombin | 2.802 |
| 5 | 1E7A | Serum albumin | 2.804 | 5 | 1SHJ | Caspase-7 | 2.793 |
| 6 | 1PMV | JNK3 | 2.789 | 6 | 1PMV | JNK3 | 2.736 |
| 7 | 2P3G | MAPKAPK2 | 2.749 | 7 | 2O65 | PIM1 | 2.723 |
| 8 | 1P49 | Steroid sulfatase | 2.739 | 8 | 1J96 | AKR1C2 | 2.722 |
AKR1C2, aldo-keto reductase family 1 member C2 (bile acid binding protein); Apo A-II, apolipoprotein A-II; CA2, carbonic anhydrase 2; CFB, complement factor B; BMP2, bone morphogenetic protein 2; DBP, vitamin D-binding protein; KIF11, kinesin-like protein; MAPKAPK2, MAP kinase-activated protein kinase 2; PIM1, serine/threonine-specific proviral integration site for Moloney murine leukemia virus; NR1A2, thyroid hormone receptor β; p-38, mitogen-activated protein kinase 14; JNK1, mitogen-activated protein kinase 8; JNK3, mitogen-activated protein kinase 10; and VEGFR2, vascular endothelial growth factor receptor 2.
MAPKAPK2, JNK1/3, CD11a, and PIM1 represent potential targets that could contribute to the direct inhibitory effects of REOLv and its primary sesquiterpenes on human neutrophil function, such as chemotaxis. For example, neutrophil arrest and migration involves integrin α-L (CD11a) [83]. In neutrophils, the major substrate of MAPKAPK2 is the leukocyte specific protein 1 (LSP1), which binds to F-actin and participates directly in cell migration [84]. Imperatorin (furocoumarin) inhibits human neutrophil migration through inhibition of JNK and Ca2+ mobilization [85]. In addition, mixed lineage kinase 3 (MLK3)-JNK signaling has been reported to play a role in the regulation of neutrophil migration [86]. Likewise, PIM kinases have been reported to promote cell migration and invasion [87].
Based on the possibility that MAPKAPK2, JNK3, and PIM1 could interfere with phagocyte migration, we evaluated the binding affinity of pure viridiflorol, curzerene, spathulenol, and germacrone toward these three kinases but did not observe any binding activity. Nevertheless, we still cannot exclude a role for integrin α-L (CD11a) as a target for viridiflorol and spathulenol in human neutrophils.
We calculated the most important physico-chemical parameters for these sesquiterpenes using SwissADME [44] (Table 7) and found that the compounds are very similar to each other in terms of many ADME properties. Nevertheless, they differed noticeably in iLogP and tPSA [88]. These descriptors are usually related to the capacity of molecules to cross cellular membranes [89]. For example, germacrene B has the highest iLogP and lowest tPSA values and was calculated to be a compound that would not permeate the blood–brain barrier (BBB).
Table 7.
Physicochemical properties of germacrene B, germacrone, curzerene, viridiflorol, and spathulenol according to SwissADME results.
| Property | Germacrene B | Germacrone | Curzerene | Viridiflorol | Spathulenol |
|---|---|---|---|---|---|
| Formula | C15H24 | C15H22O | C15H20O | C15H26O | C15H24O |
| M.W. | 204.35 | 218.33 | 216.32 | 222.37 | 220.35 |
| Heavy atoms | 15 | 16 | 16 | 16 | 16 |
| Fraction Csp3 | 0.60 | 0.53 | 0.47 | 1.00 | 0.87 |
| Rotatable bonds | 0 | 0 | 2 | 0 | 0 |
| H-bond acceptors | 0 | 1 | 1 | 1 | 1 |
| H-bond donors | 0 | 1 | 0 | 1 | 1 |
| MR | 70.68 | 70.88 | 68.74 | 68.82 | 68.34 |
| tPSA | 0.00 | 17.07 | 13.14 | 20.23 | 20.23 |
| iLogP | 3.27 | 2.97 | 3.10 | 3.08 | 3.04 |
| BBB permeation | No | Yes | Yes | Yes | Yes |
M.W., molecular weight (g/mol); MR, molar refractivity; tPSA, topological polar surface area (Å2); iLogP, lipophilicity; BBB, blood–brain barrier.
In the current studies, we evaluated effects of R. albiflorum essential oils and, more specifically, individual component compounds on innate immune cells in vitro. Since we observed potentially beneficial immunomodulatory effects and low cytotoxicity, the next step would be to evaluate the selected compounds in vivo, and these studies are being considered. Indeed, previous studies have shown that in vivo treatment with sesquiterpenes can be beneficial for various clinical problems. For example, sesquiterpenes are currently under clinical evaluation for cancer treatment (reviewed in [90,91]). Likewise, animal studies with artemisinin and its semi-synthetic sesquiterpene derivative artesunate have shown that these compounds are effective in vivo treatments using animal models of autoimmune encephalomyelitis [92] and Alzheimer’s disease [93,94]. Furthermore, the sesquiterpene huperzine A is currently used clinically to improve memory and mental function in people with Alzheimer’s disease or other neurodegenerative diseases [95]. Thus, the potential for clinical development of the sesquiterpenes identified here is clearly feasible.
4. Conclusions
Essential oils isolated from the leaves of R. albiflorum contain a high amount of sesquiterpenes (up to 91%), and these essential oils can induce human neutrophil and microglial cell Ca2+ influx, which desensitizes these cells to subsequent agonist-induced functional responses. Moreover, the major constituents of R. albiflorum leaf essential oils (viridiflorol, curzerene, spathulenol, and germacrone) exhibited the same effects, inhibiting agonist-induced Ca2+ mobilization and chemotaxis in human neutrophils and agonist-induced Ca2+ mobilization in microglial cells and FPR-transfected HL60 cells. Thus, our data provide a molecular basis to explain at least part of the beneficial therapeutic effects of R. albiflorum essential oils and component compounds and suggest that inhibition of innate immune cells by component compounds of this essential oil might have anti-inflammatory effects. Future studies are now in progress to evaluate the potential of Rhododendron essential oils as therapeutic remedies for various disorders with immune and/or inflammatory mechanisms, including Alzheimer’s disease, as well as to determine the molecular targets of the active compounds.
Author Contributions
I.A.S. and M.T.Q. conceived and designed the project. I.A.S., G.Ö., T.Ö., and L.N.K. performed the experiments. A.I.K. conducted molecular modeling. I.A.S., G.Ö., T.Ö., L.N.K., and A.I.K. analyzed and interpreted the data. I.A.S., G.Ö., A.I.K., and M.T.Q. drafted and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported in part by National Institutes of Health IDeA Program Grants GM115371 and GM103474; USDA National Institute of Food and Agriculture Hatch project 1009546; the Montana State University Agricultural Experiment Station; and the Tomsk Polytechnic University Development Program.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki and approved by the Montana State University Institutional Review Board (protocol MQ041017, approved 4/1/2017).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data that support the findings of this study are available from the authors upon reasonable request.
Conflicts of Interest
The authors declare no competing financial interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Popescu R., Kopp B. The genus Rhododendron: An ethnopharmacological and toxicological review. J. Ethnopharmacol. 2013;147:42–62. doi: 10.1016/j.jep.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 2.Jaiswal R., Jayasinghe L., Kuhnert N. Identification and characterization of proanthocyanidins of 16 members of the Rhododendron genus (Ericaceae) by tandem LC-MS. J. Mass Spectrom. 2012;47:502–515. doi: 10.1002/jms.2954. [DOI] [PubMed] [Google Scholar]
- 3.Prakash D., Upadhyay G., Singh B.N., Dhakarey R., Kumar S., Singh K.K. Free-radical scavenging activities of himalayan rhododendrons. Curr. Sci. India. 2007;92:526–532. [Google Scholar]
- 4.Gautam V., Sharma A., Arora S., Bhardwaj R., Ahmad A., Ahamad B., Ahmad P. In-vitro antioxidant, antimutagenic and cancer cell growth inhibition activities of Rhododendron arboreum leaves and flowers. Saudi J. Biol. Sci. 2020;27:1788–1796. doi: 10.1016/j.sjbs.2020.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zong L., Zhang J., Dai L., Liu J., Yang Y., Xie J., Luo X. The anti-inflammatory properties of Rhododendron molle leaf extract in LPS-induced RAW264.7. Chem. Biodivers. 2020;17:e2000477. doi: 10.1002/cbdv.202000477. [DOI] [PubMed] [Google Scholar]
- 6.Zhang J., Liu J., Dai L.F., Zhang H.Y., Chen M.N., Cai X.F., Xie J.K., Luo X.D. Unlocking the potential antioxidant and anti-inflammatory activities of Rhododendron molle G. Don. Pak. J. Pharm. Sci. 2019;32:2375–2383. [PubMed] [Google Scholar]
- 7.Guo Y., Yu X.M., Chen S., Wen J.Y., Chen Z.W. Total flavones of Rhododendron simsii planch flower protect rat hippocampal neuron from hypoxia-reoxygenation injury via activation of BKCA channel. J. Pharm. Pharmacol. 2020;72:111–120. doi: 10.1111/jphp.13178. [DOI] [PubMed] [Google Scholar]
- 8.Gautam V., Kohli S.K., Arora S., Bhardwaj R., Kazi M., Ahmad A., Raish M., Ganaie M.A., Ahmad P. Antioxidant and antimutagenic activities of different fractions from the leaves of Rhododendron arboreum Sm. and their GC-MS profiling. Molecules. 2018;23:2239. doi: 10.3390/molecules23092239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rapinski M., Musallam L., Arnason J.T., Haddad P., Cuerrier A. Adipogenic activity of wild populations of Rhododendron groenlandicum, a medicinal shrub from the James Bay Cree traditional pharmacopeia. Evid. Based Complement. Altern. Med. 2015;2015:492458. doi: 10.1155/2015/492458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang C.M., Hsu Y.M., Jhan Y.L., Tsai S.J., Lin S.X., Su C.H., Chou C.H. Structure elucidation of procyanidins isolated from Rhododendron formosanum and their anti-oxidative and anti-bacterial activities. Molecules. 2015;20:12787–12803. doi: 10.3390/molecules200712787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moreman D.E. Native American Medicinal Plants. An Ethnobotanical Dictionary. Timber Press; Portland, OR, USA: 2009. p. 799. [Google Scholar]
- 12.Zuo X., Gu Y.N., Wang C., Zhang J.R., Zhang J., Wang G.Q., Wang F. A systematic review of the anti-inflammatory and immunomodulatory properties of 16 essential oils of herbs. Evid. Based Compl. Alt. 2020;2020:8878927. doi: 10.1155/2020/8878927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reis D., Jones T. Aromatherapy using essential oils as a supportive therapy. Clin. J. Oncol. Nurs. 2017;21:16–19. doi: 10.1188/17.CJON.16-19. [DOI] [PubMed] [Google Scholar]
- 14.Rombola L., Amantea D., Russo R., Adornetto A., Berliocchi L., Tridico L., Corasaniti M.T., Sakurada S., Sakurada T., Bagetta G., et al. Rational basis for the use of bergamot essential oil in complementary medicine to treat chronic pain. Mini Rev. Med. Chem. 2016;16:721–728. doi: 10.2174/1389557516666160321113913. [DOI] [PubMed] [Google Scholar]
- 15.Di Martile M., Garzoli S., Ragno R., Del Bufalo D. Essential oils and their main chemical components: The past 20 years of preclinical studies in melanoma. Cancers. 2020;12:2650. doi: 10.3390/cancers12092650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Cassia da Silveira E.S.R., Andrade L.N., Dos Reis Barreto de Oliveira R., de Sousa D.P. A review on anti-inflammatory activity of phenylpropanoids found in essential oils. Molecules. 2014;19:1459–1480. doi: 10.3390/molecules19021459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Judzentiene A., Budiene J., Svediene J., Garjonyte R. Toxic, radical scavenging, and antifungal activity of Rhododendron tomentosum H. Essential oils. Molecules. 2020;25:1676. doi: 10.3390/molecules25071676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jesionek A., Kokotkiewicz A., Mikosik-Roczynska A., Ciesielska-Figlon K., Luczkiewicz P., Bucinski A., Daca A., Witkowski J.M., Bryl E., Zabiegala B., et al. Chemical variability of Rhododendron tomentosum (Ledum Palustre) essential oils and their pro-apoptotic effect on lymphocytes and rheumatoid arthritis synoviocytes. Fitoterapia. 2019;139:104402. doi: 10.1016/j.fitote.2019.104402. [DOI] [PubMed] [Google Scholar]
- 19.Raal A., Orav A., Gretchushnikova T. Composition of the essential oil of the Rhododendron tomentosum Harmaja from Estonia. Nat. Prod. Res. 2014;28:1091–1098. doi: 10.1080/14786419.2014.907287. [DOI] [PubMed] [Google Scholar]
- 20.Yang K., Zhou Y.X., Wang C.F., Du S.S., Deng Z.W., Liu Q.Z., Liu Z.L. Toxicity of Rhododendron anthopogonoides essential oil and its constituent compounds towards Sitophilus zeamais. Molecules. 2011;16:7320–7330. doi: 10.3390/molecules16097320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bai L., Jiao M.L., Zang H.Y., Guo S.S., Wang Y., Sang Y.L., Du S.S. Chemical composition of essential oils from four rhododendron species and their repellent activity against three stored-product insects. Environ. Sci. Pollut. Res. 2019;26:23198–23205. doi: 10.1007/s11356-019-05577-1. [DOI] [PubMed] [Google Scholar]
- 22.Dosoky N.S., Satyal P., Pokharel S., Setzer W.N. Chemical composition, enantiomeric distribution, and biological activities of rhododendron anthopogon leaf essential oil from nepal. Nat. Prod. Commun. 2016;11:1895–1898. doi: 10.1177/1934578X1601101230. [DOI] [PubMed] [Google Scholar]
- 23.Innocenti G., Dall’Acqua S., Scialino G., Banfi E., Sosa S., Gurung K., Barbera M., Carrara M. Chemical composition and biological properties of Rhododendron anthopogon essential oil. Molecules. 2010;15:2326–2338. doi: 10.3390/molecules15042326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beutler B. Innate immunity: An overview. Mol. Immunol. 2004;40:845–859. doi: 10.1016/j.molimm.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 25.Witko-Sarsat V., Rieu P., Descamps-Latscha B., Lesavre P., Halbwachs-Mecarelli L. Neutrophils: Molecules, functions and pathophysiological aspects. Lab. Investig. 2000;80:617–653. doi: 10.1038/labinvest.3780067. [DOI] [PubMed] [Google Scholar]
- 26.Fletcher S., Steffy K., Averett D. Masked oral prodrugs of Toll-like receptor 7 agonists: A new approach for the treatment of infectious disease. Curr. Opin. Investig. Drugs. 2006;7:702–708. [PubMed] [Google Scholar]
- 27.Schepetkin I.A., Kirpotina L.N., Khlebnikov A.I., Quinn M.T. High-throughput screening for small-molecule activators of neutrophils: Identification of novel n-formyl peptide receptor agonists. Mol. Pharmacol. 2007;71:1061–1074. doi: 10.1124/mol.106.033100. [DOI] [PubMed] [Google Scholar]
- 28.Reshetnikov V., Hahn J., Maueroder C., Czegley C., Munoz L.E., Herrmann M., Hoffmann M.H., Mokhir A. Chemical tools for targeted amplification of reactive oxygen species in neutrophils. Front. Immunol. 2018;9:1827. doi: 10.3389/fimmu.2018.01827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schepetkin I.A., Kushnarenko S.V., Ozek G., Kirpotina L.N., Sinharoy P., Utegenova G.A., Abidkulova K.T., Ozek T., Baser K.H., Kovrizhina A.R., et al. Modulation of human neutrophil responses by the essential oils from Ferula akitschkensis and their constituents. J. Agric. Food Chem. 2016;64:7156–7170. doi: 10.1021/acs.jafc.6b03205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ozek G., Schepetkin I.A., Utegenova G.A., Kirpotina L.N., Andrei S.R., Ozek T., Baser K.H.C., Abidkulova K.T., Kushnarenko S.V., Khlebnikov A.I., et al. Chemical composition and phagocyte immunomodulatory activity of Ferula iliensis essential oils. J. Leukoc. Biol. 2017;101:1361–1371. doi: 10.1189/jlb.3A1216-518RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schepetkin I.A., Kushnarenko S.V., Ozek G., Kirpotina L.N., Utegenova G.A., Kotukhov Y.A., Danilova A.N., Ozek T., Baser K.H., Quinn M.T. Inhibition of human neutrophil responses by the essential oil of Artemisia kotuchovii and its constituents. J. Agric. Food. Chem. 2015;63:4999–5007. doi: 10.1021/acs.jafc.5b01307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schepetkin I.A., Özek G., Özek T., Kirpotina L.N., Khlebnikov A.I., Quinn M.T. Chemical composition and immunomodulatory activity of Hypericum perforatum essential oils. Biomolecules. 2020;10:916. doi: 10.3390/biom10060916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cunningham C. Microglia and neurodegeneration: The role of systemic inflammation. Glia. 2013;61:71–90. doi: 10.1002/glia.22350. [DOI] [PubMed] [Google Scholar]
- 34.Hanisch U.K., Kettenmann H. Microglia: Active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 35.Hou K., Li G., Yu J., Xu K., Wu W. Receptors, channel proteins, and enzymes involved in microglia-mediated neuroinflammation and treatments by targeting microglia in ischemic stroke. Neuroscience. 2021;460:167–180. doi: 10.1016/j.neuroscience.2021.02.018. [DOI] [PubMed] [Google Scholar]
- 36.Fu Y., Yang J.M., Wang X.Y., Yang P., Zhao Y., Li K., Chen Y.J., Xu Y. Herbal compounds play a role in neuroprotection through the inhibition of microglial activation. J. Immunol. Res. 2018;2018:9348046. doi: 10.1155/2018/9348046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abu-Darwish M.S., Cabral C., Goncalves M.J., Cavaleiro C., Cruz M.T., Efferth T., Salgueiro L. Artemisia herba-alba essential oil from Buseirah (South Jordan): Chemical characterization and assessment of safe antifungal and anti-inflammatory doses. J. Ethnopharmacol. 2015;174:153–160. doi: 10.1016/j.jep.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 38.Xu M., Zhang X., Ren F., Yan T., Wu B., Bi K., Bi W., Jia Y. Essential oil of Schisandra chinensis ameliorates cognitive decline in mice by alleviating inflammation. Food Funct. 2019;10:5827–5842. doi: 10.1039/C9FO00058E. [DOI] [PubMed] [Google Scholar]
- 39.Li Y., Lv O., Zhou F., Li Q., Wu Z., Zheng Y. Linalool inhibits LPS-induced inflammation in BV2 microglia cells by activating Nrf2. Neurochem. Res. 2015;40:1520–1525. doi: 10.1007/s11064-015-1629-7. [DOI] [PubMed] [Google Scholar]
- 40.Ozek G., Ishmuratova M., Tabanca N., Radwan M.M., Goger F., Ozek T., Wedge D.E., Becnel J.J., Cutler S.J., Can Baser K.H. One-step multiple component isolation from the oil of Crinitaria tatarica (Less.) Sojak by preparative capillary gas chromatography with characterization by spectroscopic and spectrometric techniques and evaluation of biological activity. J. Sep. Sci. 2012;35:650–660. doi: 10.1002/jssc.201100950. [DOI] [PubMed] [Google Scholar]
- 41.Garcia-Mesa Y., Jay T.R., Checkley M.A., Luttge B., Dobrowolski C., Valadkhan S., Landreth G.E., Karn J., Alvarez-Carbonell D. Immortalization of primary microglia: A new platform to study hiv regulation in the central nervous system. J. Neurovirol. 2017;23:47–66. doi: 10.1007/s13365-016-0499-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Karaman M.W., Herrgard S., Treiber D.K., Gallant P., Atteridge C.E., Campbell B.T., Chan K.W., Ciceri P., Davis M.I., Edeen P.T., et al. A quantitative analysis of kinase inhibitor selectivity. Nat. Biotechnol. 2008;26:127–132. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- 43.Liu X., Ouyang S., Yu B., Liu Y., Huang K., Gong J., Zheng S., Li Z., Li H., Jiang H. Pharmmapper server: A web server for potential drug target identification using pharmacophore mapping approach. Nucleic. Acids Res. 2010;38:W609–W614. doi: 10.1093/nar/gkq300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Daina A., Michielin O., Zoete V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017;7:42717. doi: 10.1038/srep42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schaff U.Y., Yamayoshi I., Tse T., Griffin D., Kibathi L., Simon S.I. Calcium flux in neutrophils synchronizes β2 integrin adhesive and signaling events that guide inflammatory recruitment. Ann. Biomed. Eng. 2008;36:632–646. doi: 10.1007/s10439-008-9453-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krause K.H., Campbell K.P., Welsh M.J., Lew D.P. The calcium signal and neutrophil activation. Clin. Biochem. 1990;23:159–166. doi: 10.1016/0009-9120(90)80030-M. [DOI] [PubMed] [Google Scholar]
- 47.Richardon R.M., Ali H., Tomhave E.D., Haribabu B., Snyderman R. Cross-desensitization of chemoattractant receptors occurs at multiple levels—Evidence for a role for inhibition of phospholipase-c activity. J. Biol. Chem. 1995;270:27829–27833. doi: 10.1074/jbc.270.46.27829. [DOI] [PubMed] [Google Scholar]
- 48.Fachini-Queiroz F.C., Kummer R., Estevao-Silva C.F., Carvalho M.D., Cunha J.M., Grespan R., Bersani-Amado C.A., Cuman R.K. Effects of thymol and carvacrol, constituents of Thymus vulgaris L. essential oil, on the inflammatory response. Evid. Based Complement. Altern. Med. 2012;2012:657026. doi: 10.1155/2012/657026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Danielli L.J., de Souza T.J.T., Maciel A.J., Ferrao M.F., Fuentefria A.M., Apel M.A. Influence of monoterpenes in biological activities of Nectandra megapotamica (Spreng.) Mez essential oils. Biomolecules. 2019;9:112. doi: 10.3390/biom9030112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trevizan L.N.F., do Nascimento K.F., Santos J.A., Kassuya C.A.L., Cardoso C.A.L., Vieira M.D., Moreira F.M.F., Croda J., Formagio A.S.N. Anti-inflammatory, antioxidant and anti-mycobacterium tuberculosis activity of viridiflorol: The major constituent of Allophylus edulis (A. St.-Hil., A. Juss. & Cambess.) Radlk. J. Ethnopharmacol. 2016;192:510–515. doi: 10.1016/j.jep.2016.08.053. [DOI] [PubMed] [Google Scholar]
- 51.Gilabert M., Marcinkevicius K., Andujar S., Schiavone M., Arena M.E., Bardon A. Sesqui and triterpenoids from the liverwort Lepidozia chordulifera inhibitors of bacterial biofilm and elastase activity of human pathogenic bacteria. Phytomedicine. 2015;22:77–85. doi: 10.1016/j.phymed.2014.10.006. [DOI] [PubMed] [Google Scholar]
- 52.Wang Y.D., Li J.H., Guo J.Q., Wang Q.Y., Zhu S.G., Gao S.Y., Yang C., Wei M., Pan X.D., Zhu W., et al. Cytotoxic and antitumor effects of curzerene from Curcuma longa. Planta Med. 2017;83:23–29. doi: 10.1055/s-0042-107083. [DOI] [PubMed] [Google Scholar]
- 53.Figueiredo P.L.B., Pinto L.C., da Costa J.S., da Silva A.R.C., Mourao R.H.V., Montenegro R.C., da Silva J.K.R., Maia J.G.S. Composition, antioxidant capacity and cytotoxic activity of Eugenia uniflora L. chemotype-oils from the amazon. J. Ethnopharmacol. 2019;232:30–38. doi: 10.1016/j.jep.2018.12.011. [DOI] [PubMed] [Google Scholar]
- 54.Dos Santos E., Radai J.A.S., do Nascimento K.F., Formagio A.S.N., de Matos Balsalobre N., Ziff E.B., Castelon Konkiewitz E., Kassuya C.A.L. Contribution of spathulenol to the anti-nociceptive effects of Psidium guineense. Nutr. Neurosci. 2020:1–11. doi: 10.1080/1028415X.2020.1815330. [DOI] [PubMed] [Google Scholar]
- 55.do Nascimento K.F., Moreira F.M.F., Alencar Santos J., Kassuya C.A.L., Croda J.H.R., Cardoso C.A.L., Vieira M.D.C., Gois Ruiz A.L.T., Ann Foglio M., de Carvalho J.E., et al. Antioxidant, anti-inflammatory, antiproliferative and antimycobacterial activities of the essential oil of Psidium guineense Sw. and spathulenol. J. Ethnopharmacol. 2018;210:351–358. doi: 10.1016/j.jep.2017.08.030. [DOI] [PubMed] [Google Scholar]
- 56.Bomfim L.M., Menezes L.R., Rodrigues A.C., Dias R.B., Rocha C.A., Soares M.B., Neto A.F., Nascimento M.P., Campos A.F., Silva L.C., et al. Antitumour activity of the microencapsulation of Annona vepretorum essential oil. Basic Clin. Pharmacol. Toxicol. 2016;118:208–213. doi: 10.1111/bcpt.12488. [DOI] [PubMed] [Google Scholar]
- 57.Perez-Hernandez N., Ponce-Monter H., Medina J.A., Joseph-Nathan P. Spasmolytic effect of constituents from Lepechinia caulescens on rat uterus. J. Ethnopharmacol. 2008;115:30–35. doi: 10.1016/j.jep.2007.08.044. [DOI] [PubMed] [Google Scholar]
- 58.Riaz A., Rasul A., Kanwal N., Hussain G., Shah M.A., Sarfraz I., Ishfaq R., Batool R., Rukhsar F., Adem S. Germacrone: A potent secondary metabolite with therapeutic potential in metabolic diseases, cancer and viral infections. Curr. Drug Metab. 2020;21:1079–1090. doi: 10.2174/1389200221999200728144801. [DOI] [PubMed] [Google Scholar]
- 59.Liao Q., Qian Z., Liu R., An L., Chen X. Germacrone inhibits early stages of influenza virus infection. Antivir. Res. 2013;100:578–588. doi: 10.1016/j.antiviral.2013.09.021. [DOI] [PubMed] [Google Scholar]
- 60.He W., Zhai X., Su J., Ye R., Zheng Y., Su S. Antiviral activity of germacrone against pseudorabies virus in vitro. Pathogens. 2019;8:258. doi: 10.3390/pathogens8040258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Feng J., Bai X., Cui T., Zhou H., Chen Y., Xie J., Shi Q., Wang H., Zhang G. In vitro antiviral activity of germacrone against porcine reproductive and respiratory syndrome virus. Curr. Microbiol. 2016;73:317–323. doi: 10.1007/s00284-016-1042-8. [DOI] [PubMed] [Google Scholar]
- 62.Liu Y., Wang W., Fang B., Ma F., Zheng Q., Deng P., Zhao S., Chen M., Yang G., He G. Anti-tumor effect of germacrone on human hepatoma cell lines through inducing G2/M cell cycle arrest and promoting apoptosis. Eur. J. Pharmacol. 2013;698:95–102. doi: 10.1016/j.ejphar.2012.10.013. [DOI] [PubMed] [Google Scholar]
- 63.Liu Y.Y., Zheng Q., Fang B., Wang W., Ma F.Y., Roshan S., Banafa A., Chen M.J., Chang J.L., Deng X.M., et al. Germacrone induces apoptosis in human hepatoma HEPG2 cells through inhibition of the JAK2/STAT3 signalling pathway. J. Huazhong. Univ. Sci. Technolog. Med. Sci. 2013;33:339–345. doi: 10.1007/s11596-013-1121-z. [DOI] [PubMed] [Google Scholar]
- 64.An J.F., Sun Y., Zhang Q.L., Zhang F.L., Zhang J.L. The effects of germacrone on lipopolysaccharide-induced acute lung injury in neonatal rats. Cell Mol. Biol. 2014;60:8–12. [PubMed] [Google Scholar]
- 65.Zhong Z., Chen X., Tan W., Xu Z., Zhou K., Wu T., Cui L., Wang Y. Germacrone inhibits the proliferation of breast cancer cell lines by inducing cell cycle arrest and promoting apoptosis. Eur. J. Pharmacol. 2011;667:50–55. doi: 10.1016/j.ejphar.2011.03.041. [DOI] [PubMed] [Google Scholar]
- 66.Lim M.S., Choung S.Y., Jeong K.W. Germacrone inhibits estrogen receptor α-mediated transcription in MCF-7 breast cancer cells. Phytother. Res. 2016;30:2036–2043. doi: 10.1002/ptr.5711. [DOI] [PubMed] [Google Scholar]
- 67.Yu Z., Xu J., Shao M., Zou J. Germacrone induces apoptosis as well as protective autophagy in human prostate cancer cells. Cancer Manag. Res. 2020;12:4009–4016. doi: 10.2147/CMAR.S250522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wang Z., Zhuo F., Chu P., Yang X., Zhao G. Germacrone alleviates collagen-induced arthritis via regulating Th1/Th2 balance and NF-κB activation. Biochem. Biophys. Res. Commun. 2019;518:560–564. doi: 10.1016/j.bbrc.2019.08.084. [DOI] [PubMed] [Google Scholar]
- 69.Wu T., Yin F., Kong H., Peng J. Germacrone attenuates cerebral ischemia/reperfusion injury in rats via antioxidative and antiapoptotic mechanisms. J. Cell Biochem. 2019;120:18901–18909. doi: 10.1002/jcb.29210. [DOI] [PubMed] [Google Scholar]
- 70.Zhu X., Zhang W., Jin L., Zhang G., Yang H., Yu B. Inhibitory activities of curzerenone, curdione, furanodienone, curcumol and germacrone on Ca2+-activated chloride channels. Fitoterapia. 2020;147:104736. doi: 10.1016/j.fitote.2020.104736. [DOI] [PubMed] [Google Scholar]
- 71.Dolesjs L., Motl O., Soucek M., Herout V., Sorm F. On terpenes. CVIII. Epimeric aromadendrenes. Stereoisomerism of ledol, viridiflorol and globulol. Collect. Czech. Chem. Commun. 1960;25:1483–1491. doi: 10.1135/cccc19601483. [DOI] [Google Scholar]
- 72.Buckingham J., editor. Dictionary of Natural Products. Volume 11. CRC Press; Boca Raton, FL, USA: 1997. p. 615. [Google Scholar]
- 73.Xiang C.P., Han J.X., Li X.C., Li Y.H., Zhang Y., Chen L., Qu Y., Hao C.Y., Li H.Z., Yang C.R., et al. Chemical composition and acetylcholinesterase inhibitory activity of essential oils from piper species. J. Agric. Food Chem. 2017;65:3702–3710. doi: 10.1021/acs.jafc.7b01350. [DOI] [PubMed] [Google Scholar]
- 74.Ghavam M., Manca M.L., Manconi M., Bacchetta G. Chemical composition and antimicrobial activity of essential oils obtained from leaves and flowers of Salvia hydrangea Dc. Ex Benth. Sci. Rep. 2020;10:15647. doi: 10.1038/s41598-020-73193-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Benovit S.C., Silva L.L., Salbego J., Loro V.L., Mallmann C.A., Baldisserotto B., Flores E.M., Heinzmann B.M. Anesthetic activity and bio-guided fractionation of the essential oil of Aloysia gratissima (Gillies & Hook.) Tronc. in silver catfish Rhamdia quelen. An. Acad. Bras. Cienc. 2015;87:1675–1689. doi: 10.1590/0001-3765201520140223. [DOI] [PubMed] [Google Scholar]
- 76.Beattie K.D., Waterman P.G., Forster P.I., Thompson D.R., Leach D.N. Chemical composition and cytotoxicity of oils and eremophilanes derived from various parts of Eremophila mitchellii Benth. (Myoporaceae) Phytochemistry. 2011;72:400–408. doi: 10.1016/j.phytochem.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 77.Luciardi M.C., Perez Hernandez M.V., Muruaga N., Bardon A., Arena M.E., Cartagena E. Volatiles from subtropical Convolvulaceae that interfere with bacterial cell-to-cell communication as potential antipathogenic drugs. Evid. Based Complement. Altern. Med. 2016;2016:7890260. doi: 10.1155/2016/7890260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hulley I.M., van Vuuren S.F., Sadgrove N.J., van Wyk B.E. Antimicrobial activity of Elytropappus rhinocerotis (Asteraceae) against micro-organisms associated with foot odour and skin ailments. J. Ethnopharmacol. 2019;228:92–98. doi: 10.1016/j.jep.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 79.Chen Y.Y., Peng C.X., Hu Y., Bu C., Guo S.C., Li X., Chen Y., Chen J.W. Studies on chemical constituents and anti-hepatoma effects of essential oil from Annona squamosa L. Pericarps. Nat. Prod. Res. 2017;31:1305–1308. doi: 10.1080/14786419.2016.1233411. [DOI] [PubMed] [Google Scholar]
- 80.Xiao Z., Fan B., Niu Y., Wu M., Liu J., Ma S. Characterization of odor-active compounds of various chrysanthemum essential oils by gas chromatography-olfactometry, gas chromatography-mass spectrometry and their correlation with sensory attributes. J. Chromatogr. B. 2016;1009–1010:152–162. doi: 10.1016/j.jchromb.2015.12.029. [DOI] [PubMed] [Google Scholar]
- 81.Yu Z., Wang B., Yang F., Sun Q., Yang Z., Zhu L. Chemical compositionand anti-acetyl cholinesterase activity of flower essential oils of Artemisia annua at different flowering stage. Iran. J. Pharm. Res. 2011;10:265–271. doi: 10.1177/1934578x1000500833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McNeil M.J., Porter R.B., Williams L.A., Rainford L. Chemical composition and antimicrobial activity of the essential oils from Cleome spinosa. Nat. Prod. Commun. 2010;5:1301–1306. doi: 10.1177/1934578X1000500833. [DOI] [PubMed] [Google Scholar]
- 83.Dixit N., Kim M.H., Rossaint J., Yamayoshi I., Zarbock A., Simon S.I. Leukocyte function antigen-1, kindlin-3, and calcium flux orchestrate neutrophil recruitment during inflammation. J. Immunol. 2012;189:5954–5964. doi: 10.4049/jimmunol.1201638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Qian F., Deng J., Wang G., Ye R.D., Christman J.W. Pivotal role of mitogen-activated protein kinase-activated protein kinase 2 in inflammatory pulmonary diseases. Curr. Protein Pept. Sci. 2016;17:332–342. doi: 10.2174/1389203716666150629121324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tsai Y.F., Chen C.Y., Lin I.W., Leu Y.L., Yang S.C., Syu Y.T., Chen P.J., Hwang T.L. Imperatorin alleviates psoriasiform dermatitis by blocking neutrophil respiratory burst, adhesion, and chemotaxis through selective phosphodiesterase 4 inhibition. Antioxid. Redox Signal. 2020 doi: 10.1089/ars.2019.7835. [DOI] [PubMed] [Google Scholar]
- 86.Polesskaya O., Wong C., Lebron L., Chamberlain J.M., Gelbard H.A., Goodfellow V., Kim M., Daiss J.L., Dewhurst S. MLK3 regulates fMLP-stimulated neutrophil motility. Mol. Immunol. 2014;58:214–222. doi: 10.1016/j.molimm.2013.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bialopiotrowicz E., Gorniak P., Noyszewska-Kania M., Pula B., Makuch-Lasica H., Nowak G., Bluszcz A., Szydlowski M., Jablonska E., Piechna K., et al. Microenvironment-induced PIM kinases promote CXCR4-triggered mTOR pathway required for chronic lymphocytic leukaemia cell migration. J. Cell Mol. Med. 2018;22:3548–3559. doi: 10.1111/jcmm.13632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Daina A., Michielin O., Zoete V. Ilogp: A simple, robust, and efficient description of n-octanol/water partition coefficient for drug design using the GB/SA approach. J. Chem. Inf. Model. 2014;54:3284–3301. doi: 10.1021/ci500467k. [DOI] [PubMed] [Google Scholar]
- 89.Giner B., Lafuente C., Lapena D., Errazquin D., Lomba L. Qsar study for predicting the ecotoxicity of nades towards aliivibrio fischeri. Exploring the use of mixing rules. Ecotox. Environ. Safe. 2020;191:110004. doi: 10.1016/j.ecoenv.2019.110004. [DOI] [PubMed] [Google Scholar]
- 90.Ren Y., Yu J., Kinghorn A.D. Development of anticancer agents from plant-derived sesquiterpene lactones. Curr. Med. Chem. 2016;23:2397–2420. doi: 10.2174/0929867323666160510123255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Schepetkin I.A., Plotnikov M.B., Khlebnikov A.I., Plotnikova T.M., Quinn M.T. Oximes: Novel therapeutics with anticancer and anti-inflammatory potential. Biomolecules. 2021;11:777. doi: 10.3390/biom11060777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Thomé R., de Carvalho A.C., Alves da Costa T., Ishikawa L.L., Fraga-Silva T.F., Sartori A., de Oliveira A.L., Verinaud L. Artesunate ameliorates experimental autoimmune encephalomyelitis by inhibiting leukocyte migration to the central nervous system. CNS Neurosci. Ther. 2016;22:707–714. doi: 10.1111/cns.12561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kiss E., Kins S., Zöller Y., Schilling S., Gorgas K., Groß D., Schlicksupp A., Rosner R., Kirsch J., Kuhse J. Artesunate restores the levels of inhibitory synapse proteins and reduces amyloid-β and c-terminal fragments (ctfs) of the amyloid precursor protein in an ad-mouse model. Mol. Cell Neurosci. 2021;113:103624. doi: 10.1016/j.mcn.2021.103624. [DOI] [PubMed] [Google Scholar]
- 94.Kiss E., Kins S., Gorgas K., Orlik M., Fischer C., Endres K., Schlicksupp A., Kirsch J., Kuhse J. Artemisinin-treatment in pre-symptomatic app-ps1 mice increases gephyrin phosphorylation at ser270: A modification regulating postsynaptic gaba(a)r density. Biol. Chem. 2021 doi: 10.1515/hsz-2021-0153. [DOI] [PubMed] [Google Scholar]
- 95.Kong Y.R., Tay K.C., Su Y.X., Wong C.K., Tan W.N., Khaw K.Y. Potential of naturally derived alkaloids as multi-targeted therapeutic agents for neurodegenerative diseases. Molecules. 2021;26:728. doi: 10.3390/molecules26030728. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the authors upon reasonable request.