FIG 3.
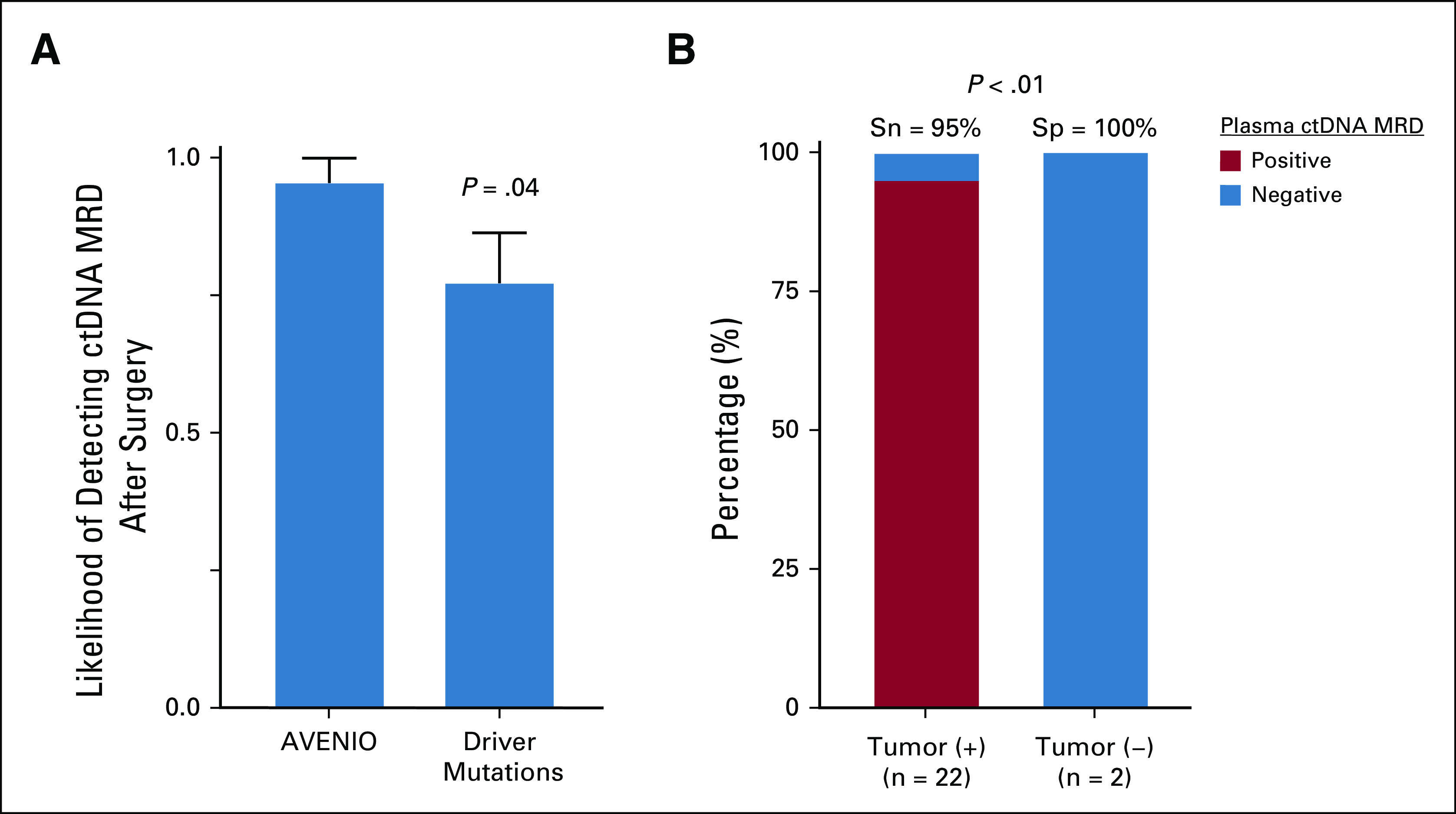
Plasma ctDNA MRD detection predicts residual disease in the surgical specimen. (A) Likelihood of detecting plasma ctDNA MRD on the day of surgery by tracking all mutations within the AVENIO target panel space or tracking only driver mutations (mean ± SEM). This figure illustrates data from the 22 patients with detectable tumor cells in the surgical specimen. P value was calculated by Student t-test. (B) Stacked bar plot depicting the sensitivity and specificity of tumor-naive plasma ctDNA MRD detection on the day of surgery according to the presence of viable tumor cells in the surgical specimen. P value was calculated by Fisher's exact test. ctDNA, circulating tumor DNA; MRD, molecular residual disease; Sn, sensitivity; Sp, specificity; Tumor (−), no viable tumor cells; Tumor (+), tumor cells present.
