Fig. 4. Lysine methylation blocks host-mediated reduction of OmpB from the bacterial surface.
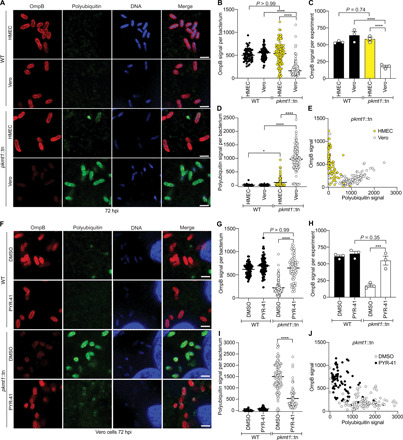
(A) HMEC and Vero cells infected WT and pkmt1::tn bacteria at 72 hpi, fixed with 4% paraformaldehyde (PFA), postfixed with methanol and stained with an anti-OmpB antibody (red) and sequentially with the FK1 antibody (green) and Hoechst (DNA; blue) (n = 3). Scale bars, 2 μm. (B) Quantification of OmpB signal per bacterium from (A) (≥63 bacteria counted). (C) Average OmpB signal per bacterium from n = 3. (D) Quantification of polyubiquitin signal on individual bacteria from (A) (≥63 bacteria). (E) OmpB and polyubiquitin signal plotted together on individual bacteria. (F) Micrographs of infected Vero cells, treated with 100 μM ubiquitin inhibitor PYR-41 between 66 and 72 hpi, stained as in (A) (n = 3). Scale bars, 2 μm. (G to J) Quantifications of (F) as in (B) to (E) (≥72 bacteria). Statistical comparisons between cell types and conditions in (B), (D), (G), and (I) were performed using a Kruskal-Wallis test with Dunn’s post hoc test and in (C) and (H) with a one-way ANOVA with Tukey’s post hoc test. ****P < 0.0001, ***P < 0.001, and *P < 0.05. All data are means ± SEM.
