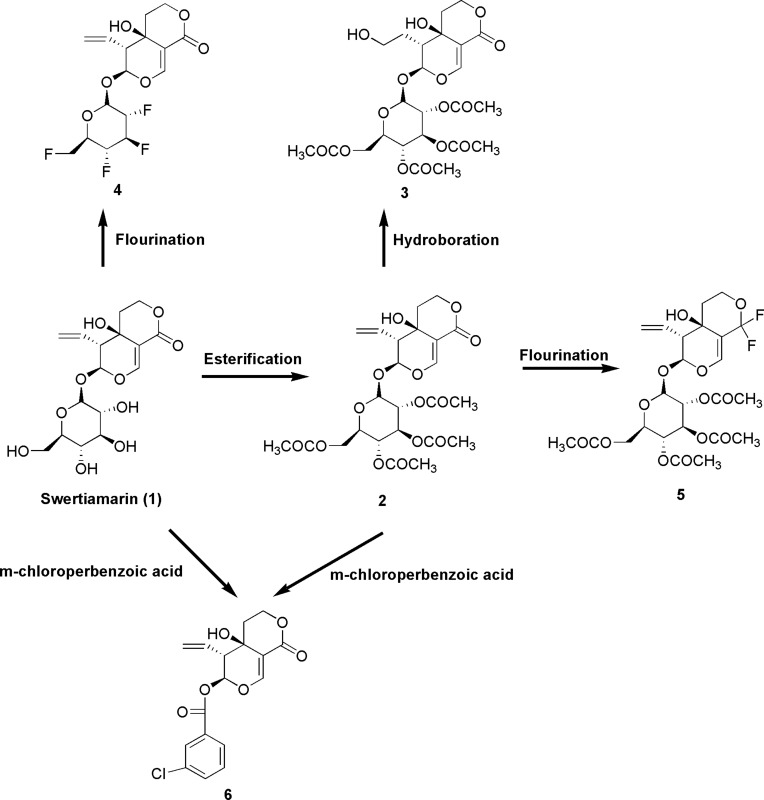
Figure 11.
Structural modifications of swertiamarin.
Notes: Esterification of glycan hydroxyl groups present in swertiamarin (1) using acetic anhydride in the presence of pyridine and dimethylformamide (DMF) to form swertiamarin tetraacetate (2). A hydroboration reaction was performed using trihydridoboron (BH3)-tetrahydrofuran (THF), hydrogen peroxide (H2O2) and NaOH to yield 3. A nucleophilic fluorination reaction was performed to attach the fluorine atom at the positions of a hydroxyl group in the glycan part of 1 using diethylaminosulphurtrifluoride (DAST) reagent to yield 4. Fluorination also takes place at the lactone group at (C-8) position 2 by using DAST reagent to form 5. When 1 and 2 were treated with m-chloroperbenzoic acid (mCPBA) for epoxidation, unexpected product 6 was formed due to anchimeric assistance.94