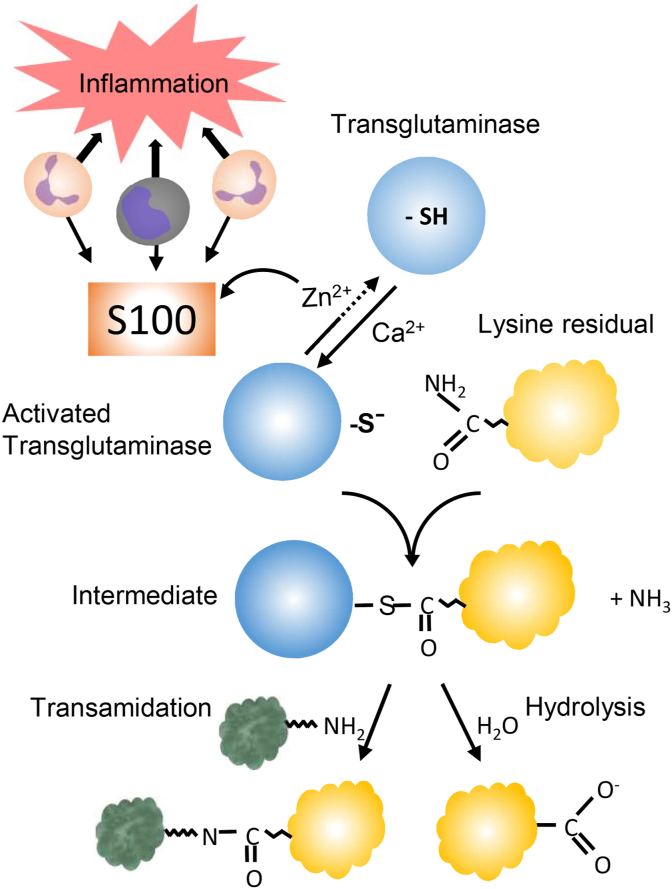
Fig. 1.
Zinc-modulated activation of transglutaminase. Leukocytes at a site of inflammation release S100-proteins which sequester Zn2+, thus facilitating the calcium-dependent activation of the enzyme. The intermediary thioester formed between the enzyme and a glutamine residue is then attacked by a primary amine, resulting in a transamidation. In the absence of an amine, water acts as the nucleophile, resulting in hydrolysis of the glutamine residue. In the transamidation, the formation of the thioester is rate limiting. In hydrolysis, the last step is rate limiting. Since the intermediate is essential for the reactions, both transamidation and hydrolysis (deamidation) are inhibited by zinc.