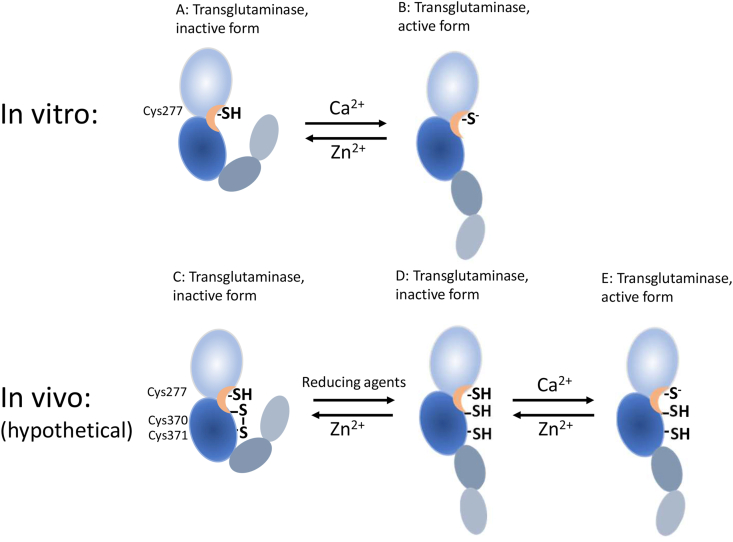
Fig. 2.
The proposed involvement of zinc in the activation of TG2. In vitro, Zn2+ competes with the binding of Ca2+ and the exposition of the active site cysteine thiol is blocked. In vivo a disulfide formed between two vicinal cysteine residues might inhibit the activation. When this disulfide is reduced, the enzyme is activated. Normal levels of Zn2+ prevents this reaction by binding to the thiol group of the reducing agent. When the concentration of active Zn2+ is reduced due to the release of S100-proteins such as calprotectin, the activation of TG2 can be completed.