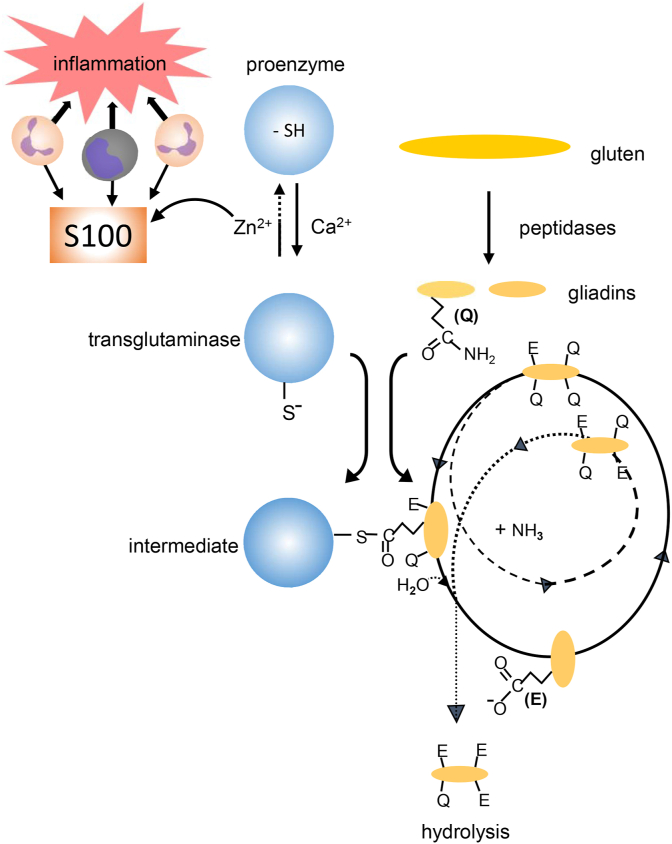
Fig. 3.
Postulated initiation of celiac disease. S100-proteins released from inflammatory cells chelate Zn2+, facilitating the calcium-dependent activation of transglutaminase type 2 (TG2). In a given order, specific glutamine residues (Q) in dietary gluten are deamidated (hydrolyzed) via TG2-catalysis, forming negatively charged glutamate residues (E). During the repeated process, a thioester intermediate is formed between the active site of the enzyme and a glutamine residue of a partly deamidated gliadin peptide. Since this complex is rather long-lived, it can function as an antigen resulting in the formation of antibodies against both deamidated gliadins and against TG2.