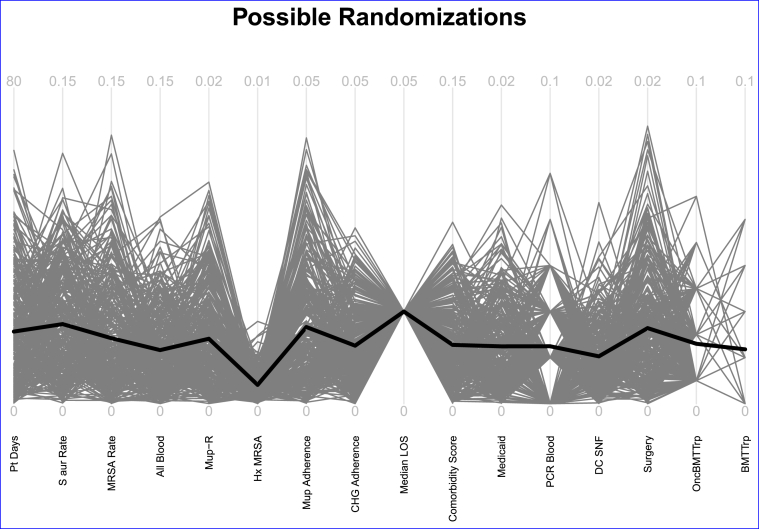
Fig. 2.
Weighting scheme used in the Mupirocin-Iodophor Swap Out Trial. The variables are: patient days (Pt days, weight = 1), Staphylococcus aureus ICU-attributable cultures per 1000 days (S aur rate, weight = 4), MRSA ICU-attributable cultures per 1000 days (MRSA rate, weight = 2), all pathogen ICU-attributable bloodstream infections per 1000 days (All Blood, weight = 4), regional mupirocin resistance estimates (Mup R, weight = 2), percent of ICU admissions with a prior history of MRSA (Hx MRSA, weight = 1), baseline usage of mupirocin (percent of mupirocin use in the first 5 days of ICU admission (Mup Adherence, weight = 1), current usage of chlorhexidine (percent adherence to daily chlorhexidine gluconate for bathing (CHG Adherence, weight = 1), median ICU length of stay (Median LOS, weight = 3), mean Elixhauser total score (Comorbidity Score, weight = 1), percent ICU patients insured by Medicaid (Medicaid, weight = 0), whether or not a facility uses polymerase chain reactions to identify MRSA in blood (PCR Blood, weight = 0), percent admissions involving a skilled nursing facility (DC SNF), percent surgical admissions (Surgery, weight = 1), whether the ICU had specialty units for oncology, bone marrow transplant, or transplant units (OncBMTTrp, weight = 2), if the ICU has bone marrow transplant or transplant units (BMTTrp, weight = 0). Note that Median LOS has the same value for all the re-randomizations. That is, for this variable, every assignment of treatment and control within the pairs results in the same mean difference in median length of stay between the control and treatment arms. This is likely due to the very small variability of this variable. The vast majority of the hospitals had the same median length of stay.