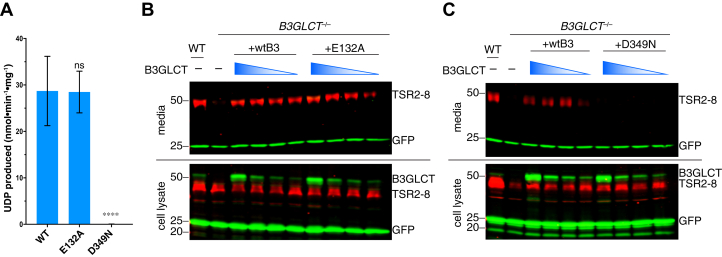
Figure 2.
The DxD motif of the C-GT domain is responsible for B3GLCT glucosyltransferase activity.A, in vitro enzyme activity assay (20 min) of WT, E132A mutant, and D349N mutant B3GLCTΔREEL with UDP-Glc as donor substrate (50 μM) and Fuc-O-TSR3 (25 μM) as acceptor substrate. Biological triplicates were performed with three different batches of enzymes. Error bars represent standard deviation, n = 3. Statistical analysis was performed with one-way ANOVA by comparing activities of the mutants to WT in Prism 7. ∗∗∗∗p < 0.0001; ns, not significant. B and C, plasmids encoding ADAMTS20 TSR2-8 (TSR2-8) and GFP were cotransfected into wild type (WT) and B3GLCT knockout (B3GLCT−/−) HEK293T cells. Rescue experiments were performed by cotransfection with a plasmid encoding full-length B3GLCT WT (wtB3), E132A mutant (B) or D349N mutant (C). Serial dilutions of B3GLCT-FL plasmids were performed starting with 0.24 μg of plasmids diluted 5-, 10-, and 20-fold. Media and cell lysates were analyzed by western blot probed with anti-Myc (red) to detect ADAMTS20 TSR2-8, anti-B3GLCT to detect endogenous or transfected B3GLCT (green, 50 kDa), and for transfection and loading control anti-GFP (green, 25 kDa).