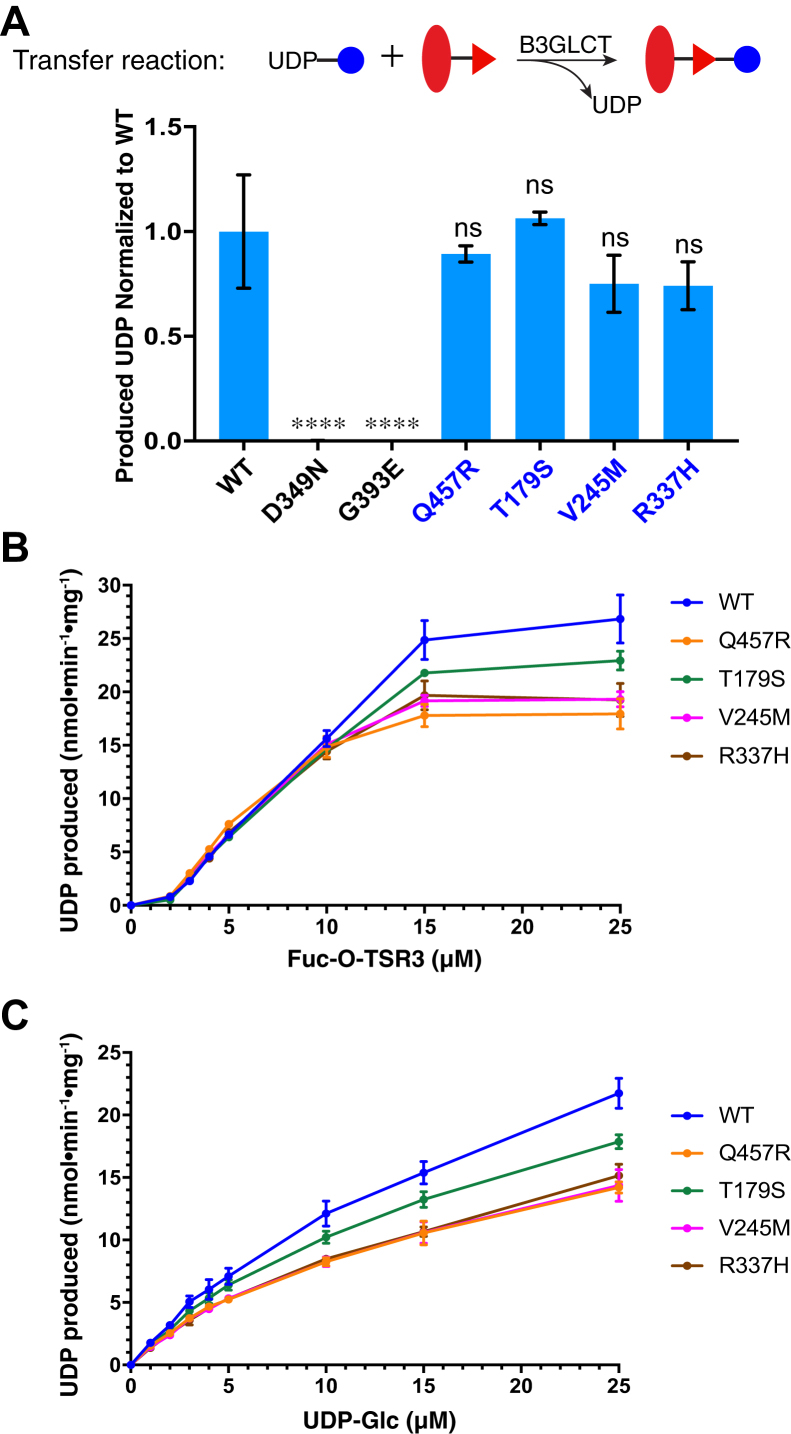
Figure 3.
PTRPLS mutants are not catalytically active, but PTRPLS-like mutants are.A, top, reaction catalyzed by B3GLCT for transfer Glc to Fuc-O-TSR3. Solid blue circle, glucose; red oval, TSR3; red triangle, fucose. A, bottom, in vitro enzyme assay (20 min) of WT, PTRPLS mutants (black), and PTRPLS-like mutants (blue). Calculated UDP produced by the mutants was normalized to the UDP produced by WT. Experiment was repeated in biological triplicates with three batches of purified enzymes. Error bars represent standard deviation, n = 3. Statistical significance was analyzed by comparison of the mutants to WT with one-way ANOVA analysis in Prism 7. ∗∗∗∗p < 0.0001; ns, not significant. B and C, substrate concentration dependent kinetics of WT and PTRPLS-like mutants with various Fuc-O-TSR3 (B) and UDP-Glc concentrations (C) (n = 3). Technical replicates with error bar show standard deviation for each point.