Highlights
-
•
Amyloid burden only slightly improved predictions of memory and executive function.
-
•
With regular cognitive assessments, amyloid-PET may not improve cognitive prognosis.
-
•
These findings support the value of routine cognitive assessments in clinical care.
Keywords: Alzheimer’s disease, amyloid-PET, Prediction, Cognitive change, Longitudinal studies
Abstract
Background
Amyloid (A) is thought to initiate a cascade of pathology culminating in Alzheimer’s disease-related cognitive decline. A accumulation in brain tissues may begin one to two decades prior to clinical diagnosis of Alzheimer’s disease. Prior studies have demonstrated that Aβ detected in vivo with positron emission tomography with amyloid ligands (amyloid-PET) predicts contemporaneously measured cognition and future cognitive trajectories. Prior studies have not evaluated the added value of A measures in predicting future cognition when repeated past cognitive measures are available. We evaluated the extent to which amyloid-PET improves prediction of future cognitive changes over and above predictions based only on sociodemographics and past cognitive measures.
Methods
We used data from participants in the University of California Davis Alzheimer’s Disease Research cohort who were cognitively normal at baseline, participated in amyloid-PET imaging, and completed at least three cognitive assessments prior to amyloid-PET imaging ( = 132 for memory and = 135 for executive function). We used sociodemographic and cognitive measures taken prior to amyloid-PET imaging to predict cognitive trajectory after amyloid-PET imaging and assessed whether measures of amyloid burden improved predictions of subsequent cognitive change. Improvements in prediction were characterized as percent reduction in the mean squared error (MSE) in predicted cognition post amyloid-PET and increase in percent variance explained.
Results
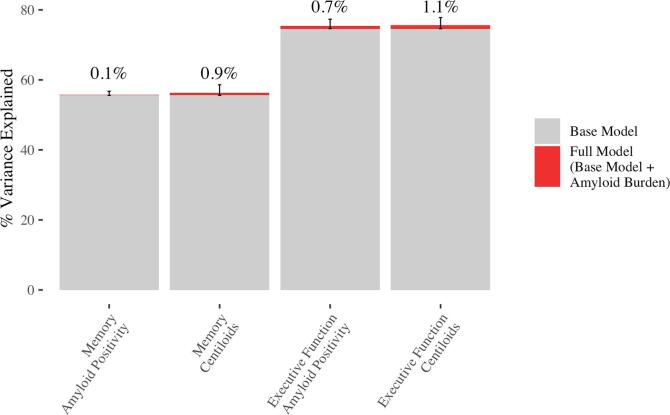
The base model using only sociodemographics and past cognitive performance explained the majority of variance in both predicted memory measures (55.6%) and executive function measures (74.5%) following amyloid-PET. Adding amyloid positivity to the model reduced the MSE for memory by 0.2%, 95% CI: (0%, 2.6%), p = 0.48 and for executive function by 3.4%, 95% CI: (0.6%, 10.2%), p = 0.002. This corresponded to an increase in the percent variance explained of 0.1%, 95% CI: (0%, 1.2%) for memory and 0.9%, 95% CI: (0.1%, 2.8%) for executive function. Similar results were obtained using a continuous measure of amyloid burden.
Conclusion
In this cohort, the addition of amyloid burden slightly improved predictions of executive function compared to models based only on past cognitive assessments and sociodemographics. When repeated cognitive assessments are available, the additional utility of amyloid-PET in predicting future cognitive impairment may be limited.
1. Introduction
Amyloid (A) plaques in brain tissue are hypothesized to be a primary causative agent of neurodegeneration in Alzheimer’s disease (Hardy and Higgins, 1992, Hardy and Selkoe, 2002, Selkoe and Hardy, 2016). Since the development of amyloid imaging probes for positron emission tomography (PET) in the early 2000s (Agdeppa et al., 2001, Mathis et al., 2002), it has been possible to visualize amyloid plaques in vivo, as well as quantify total amyloid burden in the brain (Ikonomovic et al., 2008). This technology has enabled a better understanding of the etiology of Alzheimer’s disease: it is now known that A accumulates in brain tissues beginning up to two decades prior to diagnosis and it is thought that A initiates a cascade of pathology culminating in Alzheimer’s related cognitive decline (Jack et al., 2013).
In the context of clinical applications, amyloid-PET imaging allows for the exclusion of the diagnosis of Alzheimer’s disease pre-mortem among individuals with dementia (Marcus et al., 2014), and may be useful in the development of therapies that specifically target amyloid (Vlassenko et al., 2012). However, the clinical value of amyloid-PET imaging data depends largely on its prognostic capability for outcomes of relevance to patients, such as memory complaints.
Prior research shows an independent association between amyloid burden and cognition (e.g. Hanseeuw et al., 2019, Bouter et al., 2019), amyloid burden and cognitive decline (Doraiswamy et al., 2014, Donohue et al., 2017, Vemuri et al., 2015, Mormino et al., 2014, Bouallègue et al., 2017), as well as future mild cognitive impairment and Alzheimer’s disease diagnoses (Rowe et al., 2013). Most prior research includes individuals who are cognitively normal at baseline (e.g. Rowe et al., 2013, Donohue et al., 2017). While amyloid imaging remains primarily used in research settings and tau is a more proximal biomarker to cognitive decline (Nelson et al., 2012; La Joie et al., 2020), there is significant interest in determining whether amyloid is a reliable biomarker for cognitive decline. If amyloid-PET were such a biomarker, it would strengthen the justification for its use in clinical settings. Recent research has been oriented towards evaluating amyloid-PET’s prognostic ability in clinical settings (e.g. Shea et al., 2018) and evaluating prognostic differences of various PET-imaging protocols (e.g. Morris et al., 2016). A large randomized trial is underway to evaluate the effects of incorporating amyloid-PET imaging into diagnostic criteria (Dubois et al., 2014). Early results of this trial indicate that in individuals with mild cognitive impairment or dementia of uncertain etiology, the use of amyloid-PET was associated with changes in clinical management (Rabinovici et al., 2019).
However, much of the information that might be derived from A may be accessible via simpler measures, such as past cognitive trajectories (Leuzy et al., 2014). This possibility is consistent with recent findings that subtle cognitive deficits predict future changes in amyloid (Aschenbrenner et al., 2018). Because amyloid-PET imaging is expensive, evaluating whether such imaging offers novel information that could not be attained via less costly methods is important (Leuzy et al., 2014). Prior studies do not address the clinically relevant goal of predicting future changes in cognition when, in addition to multiple cognitive assessments, basic demographic characteristics are available–information already typically available in a clinical setting. Such prognostic information is likely to be important to patients and family members and may inform clinical decision making. As amyloid-PET becomes more widely available, cognitively unimpaired older adults may seek out imaging to help understand subjective memory complaints or anticipate future cognitive changes (Langa and Burke, 2019). It is thus critical to evaluate whether amyloid-PET provides improved understanding of future cognitive change. In this context, understanding the prognostic capability of amyloid-PET is important.
Using data from cognitively normal participants at baseline from the University of California Davis Alzheimer’s Disease Research Center (UC-ADRC) diversity cohort (Hinton et al., 2010), we evaluated whether measures of amyloid burden in the brain improved predictions of subsequent cognitive measures beyond predictions based exclusively on prior cognitive assessments and demographic information.
2. Methods
2.1. Data Source and Measures
The UC-ADRC cohort included 154 participants who were cognitively normal at baseline and underwent amyloid-PET imaging during followup; study design has previously been described in detail (Han et al., 2020a). All individuals were cognitively normal at the time of amyloid-PET. In addition to clinic-based recruitment, a variety of community-based outreach methods were employed to obtain a racially and ethnically diverse group of participants, as well as participants with a range of educational backgrounds. All participants were over 60 years of age at enrollment; individuals with unstable major medical illness, major primary psychiatric disorder, or substance abuse or dependence in the last 5 years were excluded. Participants received a thorough multidisciplinary clinical evaluation which included detailed medical history, neurological examination, laboratory tests, and neuropsychological testing using the Uniform Data Set battery (Weintraub et al., 2009, Morris et al., 2006). Diagnosis of cognitive status (normal, MCI, or dementia) was made according to standard criteria and methods (Morris et al., 2006). Participants received repeated assessments approximately yearly and the current analysis was restricted to individuals with at least three cognitive assessments. The amyloid-PET study was approved by the institutional review board at University of California Davis and all study participants provided written informed consent.
Amyloid-PET imaging used [11C]Pittsburgh compound B (PiB) or Florbetapir (18F). PiB-PET images were completed at the Lawrence Berkeley National Laboratory on a Siemens ECAT EXACT HR PET scanner in 3D acquisition model. PiB radiotracer was synthesized at this facility using a standard protocol where 10 to 15 mCi of [11C] PiB was injected into an antecubital vein (Mathis et al., 2003). Dynamic acquisition frames (34 to 35 frames total) were obtained over 90 min. AV45 scans were acquired on a Siemen’s Biograph mCT 40PET machine during a 50- to 70-minute interval following a 10 mCi (370 MBq) bolus injection of Florbetapir (18F).
PiB data were preprocessed with procedures previously described using a gray matter cerebellar reference region to calculate distribution volume ratio (DVR) images (Marchant et al., 2013). The Global PiB Index was generated from the mean DVRs from regions of interest vulnerable to early A deposition, which include the frontal cortex (anterior to the precentral gyrus), lateral parietal cortex, lateral temporal cortex, posterior cingulate, and precuneus. Florbetapir data were analyzed using standard uptake value ratio (SUVr) measures (Landau et al., 2013). Four five-minute frames 50–70 min after injection were averaged and the image data was spatially normalized to a standard anatomical atlas in our laboratory. Mean tracer retention was calculated from six predefined target cortical regions of interest (medial orbital frontal, temporal, parietal, anterior cingulate, posterior cingulate, and precuneus) and whole cerebellar gray matter reference region, based on T1-weighted high-resolution MRI images. (All MRIs were obtained at the Imaging Research Center at UC Davis: 56% were obtained on a Siemen’s 3 T Tim Trio and 39% were obtained on a GE 1.5 machine. All PET analysis was done in each subject’s native space, using the alignment of PET image to closest date structural MRI.) Participants were determined to be amyloid-positive using published DVR or SUVR thresholds according to each radiotracer (a PiB cutoff of 1.10 and a florbetapir cutoff of 1.47 were used; see Marchant et al., 2013, Landau et al., 2013 for additional details).
Memory and executive function scores were obtained for each assessment, including assessments prior to amyloid-PET and assessments after amyloid-PET. Two different neuropsychological test batteries contributed to theses cognitive test scores: the Spanish-English Neuropsychological Assessment Scales (SENAS) (Mungas et al., 2004, Mungas et al., 2005) and an alternative cognitive battery for participants who were enrolled into a cohort examining ischemic vascular contributions to cognitive decline and dementia. The tests used to derive these measures are described in the supplemental information (Table 1). Item response theory methods were used to create harmonized cognitive measures with equated metrics so that results from the two test batteries could be combined in analyses. The test harmonization process is described in a previous publication (Han et al., 2020b) and details on the methods and results are given as part of the technical appendix. Scores from both batteries were on a common standard score metric (mean = 0, standard deviation = 1) referenced to a diverse sample of more than 400 community dwelling English and Spanish speaking older adults. As sensitivity analysis, we repeated the primary analysis with only scores derived from SENAS measures. The results of this sensitivity analysis are given in the Supplemental Information.
Table 1.
Summary of training and testing data for memory and executive function. Not all participants were used in both the training and testing data since three cognitive assessments were required pre-amyloid-PET to be in the training data, and at least one cognitive assessment was required post-amyloid-PET to be in the testing data. SD = standard deviation.
| Memory |
Executive Function |
|||||
|---|---|---|---|---|---|---|
| Overall | Testing Data | Training Data | Overall | Testing Data | Training Data | |
| Number | 132 | 126 | 66 | 135 | 129 | 66 |
| Mean Years of Education | 14.6 | 14.9 | 14 | 14.5 | 14.8 | 14 |
| SD Years of Education | 3.8 | 3.8 | 4 | 3.9 | 3.9 | 4 |
| Mean Number of Assessments | 5.6 | 4.8 | 6.9 | 5.8 | 5 | 7.4 |
| SD Number of Assessments | 3.3 | 3.3 | 2.8 | 3.5 | 3.5 | 2.9 |
| Percent Amyloid Positive | 23% | 25% | 20% | 24% | 26% | 20% |
| Hispanic | 27% | 22% | 35% | 27% | 22% | 35% |
| African American | 16% | 14% | 20% | 16% | 15% | 20% |
| Asian | 7% | 9% | 5% | 7% | 9% | 5% |
| Other | 1% | 1% | 0% | 1% | 1% | 0% |
| White | 49% | 54% | 41% | 49% | 53% | 41% |
2.2. Analysis
Fig. 1 gives a flowchart of the analysis, designed with the following two goals in mind: to leverage all past information on individuals with varying numbers of cognitive assessments in a way that was consistent across individuals and over time, and to use all post-PET scan cognitive assessments to evaluate the added value of amyloid burden. To achieve the first goal, we first estimated mixed-effects models to derive estimated values for cognition for the previous, current, and subsequent visits; the estimated values from these mixed-effects models were then used as independent variables in a more complex model trained to optimally predict cognitive scores, prior to the amyloid-PET scan. Finally, we used this optimized model to predict cognitive scores after amyloid-PET imaging and evaluated whether information on amyloid burden improved prediction of cognition at the subsequent visit over and above the predictions based on all prior cognitive function and demographics. Two sets of analyses were run with the below procedure: one with executive function as the cognitive outcome and a second with memory as the cognitive outcome. Additional details on each step of the analysis is given below.
Fig. 1.
Flowchart of the analysis, which leverages all past information on individuals with varying numbers of cognitive assessments in a way that was consistent across individuals and over time and uses all post-PET scan cognitive assessments to evaluate the added value of amyloid burden.
2.2.1. Initial Mixed-Effects Models: Estimates of Cognition Based Soley on Past Cognitive Measures
Since the number of cognitive assessments and timing of these assessments relative to the amyloid-PET scan varies considerably, we developed a procedure to extract information about each person’s observed trajectory in a way that was consistent between people and over time. To estimate the predicted cognitive trajectory for each individual as of visit (i.e., predicting visits 1 through , with ), we estimated a linear mixed-effects model with the following two predictors: time since baseline visit and an indicator variable for the first visit to account for practice effects with individual-level random intercepts and random slopes with respect to time. After estimating this mixed-effects model using data for visits 1 to , the model fits were used to estimate predicted scores for the two most recent visits and visits as well as the subsequent visit. This procedure was repeated to get predictions for each subsequent visit for which there was cognitive data for at least one individual.
2.2.2. Variable Selection Procedure: Applying LASSO to Cognitive Trajectories Predicted by the Mixed-Effects Model and Coviarates
Using data for individuals with at least three cognitive assessments prior to their PET scan (training data), we trained a time-series regression model to predict cognitive score at visit using the following predictors: estimated score for visit , the deviations of estimated scores from the true scores for the two most recent visits ( and ) and the interactions of these deviations with time since visit , orthogonal polynomials (Kennedy and Gentle, 2018) for age and education of degrees 1–3, gender, and race/ethnicity. We selected a set of predictors that minimized the total mean squared error in cognitive predictions for held-out individuals using LASSO regression (Tibshirani, 1996) with a ten-fold cross-validation procedure. This step was conducted using only assessments prior to amyloid-PET imaging.
2.2.3. Evaluation of the Added-Value of Amyloid Burden
Once we chose the optimal prediction model for the pre-PET scan data, we used the selected predictors in a time-series regression model to predict the post-PET scan cognitive scores. For the post-PET scan data (testing data), we then assessed whether amyloid positivity, interacted with the time since the scan was performed, improved predictions of the subsequent cognitive measures post-PET. This was done by evaluating whether the addition of amyloid positivity to these models improved cognitive predictions. We evaluated this using the mean squared error (MSE) and bootstrapped confidence interval and -value using 2,000 replicates. The bootstrapped -value was obtained from the fraction of coefficients for amyloid positivity greater than zero, multiplied by two to obtain a two-sided -value and accounts for the fact that the null hypothesis on the boundary (Fitzmaurice et al., 2012). We used Pearson’s correlation coefficients to evaluate the strength of association between predictions made with and without incorporation of amyloid into the model. This analysis was repeated using approximate centiloids, calculated using formulas presented in Bourgeat et al. (2018) (Table 3; standard SPM pipeline) from raw DVRs and SUVRs.
To provide a comparison for the magnitude of the effect estimates for amyloid positivity, we also fit a linear random-effects model with a random intercept for each individual and a random slope for time in follow-up to obtain expected post-PET cognitive decline for memory and executive function measures. We compared the average rate of change estimated from this simple linear mixed-effects model to the coefficient for amyloid positivity predicting future cognition with simultaneous adjustment for prior information on cognition and covariates.
These methods were pregistered in December of 2019 and prior to submission for publication (Ackley et al., 2021).
3. Results
Table 1 summarizes the sociodemographic characteristics of individuals in the overall sample and the training and testing datasets. For memory, 132 participants contributed an average of 6.8 (standard deviation = 3.2) assessments over 7 (standard deviation = 3.9) years of follow-up and underwent PET imaging to assess amyloid uptake (SUVr) on average 3.3 (standard deviation = 3.8, median = 2.4, IQR = 5.9) years after earliest cognitive assessment. For executive function, 135 participants contributed an average of 7 (standard deviation = 3.4) assessments over 6.9 (standard deviation = 3.9) years of follow-up and underwent PET imaging to assess amyloid uptake (SUVr) on average 3.3 (standard deviation = 3.7, median = 2.1, IQR = 5.8) years after earliest cognitive assessment. The sociodemographic characteristics were similar across the training and testing sub-samples. PET scans were performed with PiB for 42% participants undergoing amyloid-PET. Approximately one-quarter of participants were amyloid positive (25% of participants who contributed memory data and 26% of participants who contributed executive function data; 40% of participants with the radiotracer PiB and 10% of participants with the radiotracer Florbetapir). In the memory data, the most recent cognitive test at least 3 months prior to the PET scan was on average 0.89 years prior (standard deviation of 0.52). In the executive function data, the most recent cognitive test at least 3 months prior to the PET scan was on average 0.87 years prior (standard deviation of 0.49).
The base model for memory included the following variables: gender, Hispanic ethnicity, predicted cognition for the current visit, and linear years of education. The base model for executive function included the following variables: Hispanic ethnicity, predicted cognition for the current visit, quadratic age, and linear years of education. The base model for memory explained 55.6% of the variance in future post-PET memory scores. The base model for executive function explained 74.5% of the variance in future post-PET executive function scores. Demographic characteristics account for a minority of the variance explained: 44.7% of the total variance (80.4% of explained variance) is explained with past cognitive measures for memory. Similarly, 68.2% of the total variance (91.5% of explained variance) is explained with past cognitive measures for executive function.
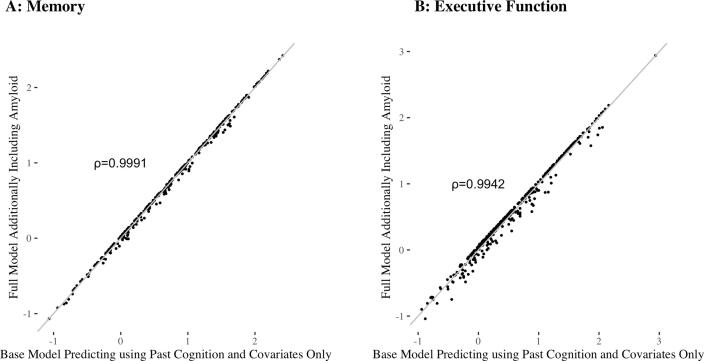
Adding amyloid positivity to the models for memory and executive function slightly improved predictions of future cognition, with larger improvements for predictions of executive function. The addition of amyloid positivity reduced the MSE in predictions of memory by 0.2%, 95% CI: (0%, 2.6%), p = 0.48, and reduced the MSE in predictions of executive function by 3.4%, 95% CI: (0.6%, 10.2%), p = 0.002. This corresponds to an increase in the percent variance explained of 0.1%, 95% CI: (0%, 1.2%) for memory and 0.9%, 95% CI: (0.1%, 2.8%) for executive function (see Fig. 2). As shown in Fig. 3, predictions from the base model and full model are highly correlated, and we obtain the following Pearson’s correlation coefficients: 0.9991 for memory and 0.9942 for executive function.
Fig. 2.
Percent variance explained for the base and full models for memory and executive function, where the full model refers to the base model with the addition of amyloid burden (amyloid positivity or approximate centiloids). Red segments show the increase in variance explained with the addition of amyloid burden, and error bars correspond to the 95% CI for the increase in the percent variance explained. Annotations (above the error bars) give the increase in variance explained. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 3.
Comparison of the base model to the full model, where the full model refers to the base model with the addition of amyloid positivity A: Base model memory predictions versus full model memory predictions. B: Base model executive function predictions versus full model executive function predictions. For both graphs, the line is shown in grey to indicate the extent of agreement between the two models.
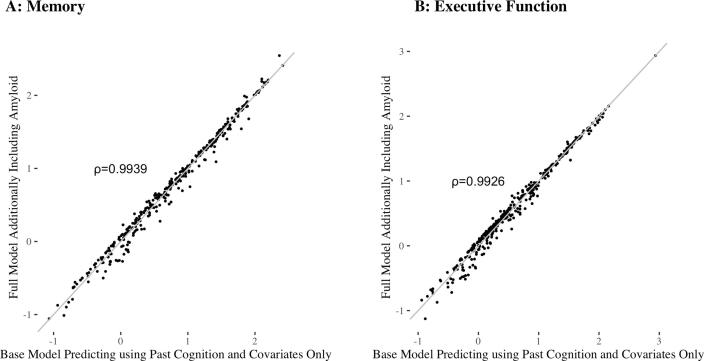
Similar results were obtained with approximate centiloids. Adding approximate centiloids to the models for memory and executive function slightly improved predictions of future cognition, with larger improvements for predictions of executive function. The addition of approximate centiloids reduced the MSE in predictions of memory by 1.5%, 95% CI: (0%, 6.7%), p = 0.095, and reduced the MSE in predictions of executive function by 4.4%, 95% CI: (0.6%, 12.9%), p = 0.003. This corresponds to an increase in the percent variance explained of 0.7%, 95% CI: (0%, 3%) for memory and 1.1%, 95% CI: (0.1%, 3.3%) for executive function (see Fig. 2). As shown in Fig. 4, predictions from the base model and full model are highly correlated, and we obtain the following Pearson’s correlation coefficients: 0.9939 for memory and 0.9926 for executive function.
Fig. 4.
Comparison of the base model to the full model, where the full model refers to the base model with the addition of approximate centiloids. A: Base model memory predictions versus full model memory predictions. B: Base model executive function predictions versus full model executive function predictions. For both graphs, the line is shown in grey to indicate the extent of agreement between the two models.
Using a random-effects model to estimate rate of cognitive change after amyloid-PET, memory declined by −0.055, 95% CI: (-0.091, −0.019) standard deviations per year and executive function declined by −0.059, 95% CI: (−0.083, −0.035) standard deviations per year. In the full model, amyloid positivity was associated with an additional annual change in memory of −0.019, 95% CI: (−0.085, 0.034) and in executive function of −0.041, 95% CI: (−0.079, −0.015) standard deviations per year. Amyloid positivity was thus associated with approximately a 33.7% of average post-PET decline in memory and a 69.7% of average post-PET decline in executive function.
4. Discussion
Compared to models based only on longitudinal cognitive assessments and demographics, the addition of amyloid burden offered small improvements in predictions of future memory and executive function. The correlations of predictions based only on prior cognition and covariates with predictions additionally incorporating amyloid were nearly perfect for both memory and executive function.
Our results suggest that when predicting future cognitive trajectories, the added value of amyloid-PET over and above past cognitive and sociodemographic measures is small in individuals for whom repeated past cognitive assessments are available. This finding highlights the clinical value of repeated cognitive assessments over time to understand likely future trajectory of cognition. While it is not necessarily surprising that past cognition already reflects the presence of the pathology that amyloid-PET is measuring or downstream pathology such as tau, it is important to understand whether amyloid-PET can provide an informative supplement to cheaper and less invasive cognitive testing to anticipate future cognitive changes.
Amyloid measures are associated with dementia and mild-cognitive impairment diagnoses (e.g. Johnson et al. (2013)) and it has been demonstrated that amyloid-PET measures can predict future cognitive trajectories (e.g. Aschenbrenner et al. (2018)) and AD diagnosis (Forsberg et al., 2008). However, prior research did not evaluate the predictive value of amyloid-PET beyond what could be gleaned based on past cognitive trajectory and basic sociodemographic variables. Since past cognitive history would often be available in a clinical care setting, the potential contribution of amyloid-PET for predicting future cognition independently of these factors is important to assess. Evaluating the contribution of amyloid-PET is particularly important because it is expensive; this expense would be justified only if it provides sufficient additional information of value to patients or significantly improves clinical management of Alzheimer’s disease and dementia (Weidman et al., 2017). Evidence on whether patient-centered outcomes are improved is forthcoming from the IDEAS trial (Rabinovici et al., 2015), but it is also important to know whether amyloid-PET imaging can provide a more accurate source of information on future cognitive changes. Previous work has indicated that amyloid positivity is associated with a higher probability of future cognitive decline, but that this decline can take several years to manifest and differences between amyloid positive and negative groups may not be clinically significant (Donohue et al., 2017). This work corroborates and extends those findings to a setting where past cognitive assessments are available.
This analysis had several strengths. The UC-ADRC is a diverse cohort with participants recruited from the clinic and community. Our method leveraged all past cognitive assessments by using estimates from a mixed-effects model (i.e. taking into account past cognition and past cognitive decline) as a predictor of cognition after amyloid-PET was completed. Typically an investigator-selected model is used to evaluate the added value of amyloid burden. However, we employed a cross-validation approach to avoid over- or underfitting the data. A data-driven variable selection procedure was used to select an optimal model that improved predictions of observations from held out individuals. With this approach, we obtained an accurate evaluation of the improvement in model fit achieved with the addition of amyloid burden.
The relatively small sample size of this study was an important limitation, although the confidence intervals for our estimated improvements in model fit exclude large improvements in the MSE. In other words, our confidence intervals suggest that even if we had a larger sample in the UC-ADRC cohort, large improvements in prediction from incorporating amyloid burden would be unlikely. The racial/ethnic diversity of our participant sample is a major strength of this analysis, but our sample was too small to permit us to formally evaluate heterogeneity across race/ethnicity, education, or other important plausible modifiers. Furthermore, our analysis only included individuals who completed at least 3 cognitive assessments prior to PET scan, which may limit generalizability to individuals who are unable or uninterested in completing cognitive assessments. Selection processes are typical in neuroimaging studies, and have been particularly problematic because a large majority of neuroimaging evidence is based on predominantly non-Hispanic white, highly educated, samples. The ADRC cohort achieved notable racial/ethnic diversity and our analysis retained this diversity, despite the potential selection. Since this sample was cognitively normal at baseline, these results may not be generalizable to populations with cognitive impairment. Cognitive change over time would likely be larger in a sample with more impairment at baseline. Evaluating these patterns in larger samples with greater cognitive and sociodemographic heterogeneity is a high priority for future research. Another limitation is that a single amyloid-PET scan does not capture the dynamics of the accumulation of amyloid. For example, if speed of accumulation is associated with subsequent neurodegeneration and cognitive decline, a single PET scan would fail to capture important information about rate. Our findings in a sample of adults who were cognitively normal at baseline are important, however, especially given the interest in using amyloid burden as an inclusion criterion in clinical trials of Alzheimer’s disease therapies. Cognitively normal, amyloid positive individuals may be the most likely to respond to disease modifying therapies and understanding the impact of amyloid on cognitive trajectories can be used to power future therapeutic trials.
Future work includes extending these analyses to other cohorts, particularly to cohorts with a significant number individuals with mild cognitive impairment and Alzheimer’s disease, and to other in-vivo neuroimaging outcomes, such as MRI or tau-PET. Incorporation of past cognitive decline into prognostic models of other biomarkers may similarly limit their clinical utility.
5. Conclusions
In this cohort, the addition of amyloid burden slightly improved predictions of executive function measures and memory, compared to models based only on longitudinal cognitive assessments and demographics. These findings may indicate that, in settings with routine cognitive assessment of cognitively normal individuals, amyloid-PET does not provide significant additional benefit for more accurate predictions of cognitive decline. These findings also support the value or routine cognitive assessments in clinical care for older adults.
CRediT authorship contribution statement
Sarah F. Ackley: Conceptualization, Methodology, Formal analysis, Visualization, Writing - original draft. Eleanor Hayes-Larson: Formal analysis, Writing - original draft. Willa D. Brenowitz: Formal analysis, Methodology, Writing - original draft. Kaitlin Swinnerton: Formal analysis, Methodology, Writing - original draft. Dan Mungas: Methodology, Writing - original draft, Writing - review & editing. Evan Fletcher: Methodology, Writing - review & editing, Data curation, Resources. Baljeet Singh: Methodology, Resources, Writing - review & editing. Rachel A. Whitmer: Methodology, Writing - review & editing, Visualization. Charles DeCarli: Conceptualization, Resources, Funding acquisition, Methodology, Writing - original draft, Supervision. M. Maria Glymour: Conceptualization, Methodology, Formal analysis, Visualization, Funding acquisition, Supervision, Writing - original draft.
Acknowledgments
This work was supported in part by NIA R01AG057869 (SA,MMG), P30 010129, R01 AG047827, R01 AG 031563 (CD), RF1 AG050782 , RF1 AG052132 (RW) and R00AG053410 (EHL).
Footnotes
Analysis Using Only SENAS Measures of Memory
In a sensitivity analysis using only SENAS memory measures and the same procedures, we obtain similar results, retaining 753 out of 894 total cognitive assessments and 117 out of 132 individuals. The variables selected were gender, African American race, predicted cognition, cubic age, and linear years of education. The addition of amyloid positivity reduced the MSE in predictions of memory by 0%, 95% CI: (0%, 0.9%), p = 0.86. This corresponds to an increase in the percent variance explained of 0%, 95% CI: (0%, 3%). The addition of approximate centiloids reduced the MSE in predictions of memory by 0.5%, 95% CI: (0%, 3.4%), p = 0.21. This corresponds to an increase in the percent variance explained of 0.2%, 95% CI: (0%, 1.1%). (Note: Due to limited observations, percentile bootstrap confidence intervals were used for the addition of amyloid positivity.)
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2021.102713.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Ackley S., Glymour M., DeCarli C. Amyloid Burden as a Predictor of Future Decline in Memory and Executive Function Measures. OSF. 2021 osf.io/7yd2t. [Google Scholar]
- Agdeppa E.D., Kepe V., Liu J., Flores-Torres S., Satyamurthy N., Petric A., Cole G.M., Small G.W., Huang S.C., Barrio J.R. “Binding Characteristics of Radiofluorinated 6-Dialkylamino-2-Naphthylethylidene Derivatives as Positron Emission Tomography Imaging Probes for Beta-Amyloid Plaques in Alzheimer’s Disease”. The Journal of Neuroscience: The Official Journal of the Society for. Neuroscience. 2001;21(24):RC189. doi: 10.1523/JNEUROSCI.21-24-j0004.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aschenbrenner A.J., Gordon B.A., Benzinger T.LS., Morris J.C., Hassenstab J.J. Influence of Tau Pet, Amyloid Pet, and Hippocampal Volume on Cognition in Alzheimer Disease. Neurology. 2018;91(9):e859–e866. doi: 10.1212/WNL.0000000000006075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouallègue F.B., Mariano-Goulart D., Payoux P., Alzheimer’s Disease Neuroimaging Initiative (ADNI, and others Comparison of Csf Markers and Semi-Quantitative Amyloid Pet in Alzheimer’s Disease Diagnosis and in Cognitive Impairment Prognosis Using the ADNI-2 Database. Alzheimer’s Research & Therapy. 2017;9(1):32. doi: 10.1186/s13195-017-0260-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeat Pierrick, Doré Vincent, Fripp Jurgen, Ames David, Masters Colin L, Salvado Olivier, Villemagne Victor L, Rowe Christopher C, AIBL research group, and others Implementing the Centiloid Transformation for 11C-Pib and β-Amyloid 18F-Pet Tracers Using Capaibl. Neuroimage. 2018;183:387–393. doi: 10.1016/j.neuroimage.2018.08.044. [DOI] [PubMed] [Google Scholar]
- Bouter Caroline, Vogelgsang Jonathan, Wiltfang Jens. Comparison Between Amyloid-Pet and Csf Amyloid-β Biomarkers in a Clinical Cohort with Memory Deficits. Clin. Chim. Acta. 2019;492:62–68. doi: 10.1016/j.cca.2019.02.005. [DOI] [PubMed] [Google Scholar]
- Donohue Michael C, Sperling Reisa A, Petersen Ronald, Sun Chung-Kai, Weiner Michael W, Aisen Paul S. Association Between Elevated Brain Amyloid and Subsequent Cognitive Decline Among Cognitively Normal Persons. JAMA. 2017;317(22):2305–2316. doi: 10.1001/jama.2017.6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doraiswamy P Murali, Sperling R.A., Johnson K., Reiman Eric M, Wong T.Z., Sabbagh M.N., Sadowsky Carl H. Florbetapir F 18 Amyloid Pet and 36-Month Cognitive Decline: A Prospective Multicenter Study. Mol. Psychiatry. 2014;19(9):1044–1051. doi: 10.1038/mp.2014.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois Bruno, Feldman Howard H, Jacova Claudia, Hampel Harald, Molinuevo José Luis, Blennow Kaj, DeKosky Steven T. Advancing Research Diagnostic Criteria for Alzheimer’s Disease: The IWG-2 Criteria. The Lancet Neurology. 2014;13(6):614–629. doi: 10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]
- Fitzmaurice Garrett M, Laird Nan M, Ware James H. Vol. 998. John Wiley & Sons; 2012. (Applied Longitudinal Analysis). [Google Scholar]
- Forsberg Anton, Engler Henry, Almkvist Ove, Blomquist Gunnar, Hagman Göran, Wall Anders, Ringheim Anna, Långström Bengt, Nordberg Agneta. PET Imaging of Amyloid Deposition in Patients with Mild Cognitive Impairment. Neurobiol. Aging. 2008;29(10):1456–1465. doi: 10.1016/j.neurobiolaging.2007.03.029. [DOI] [PubMed] [Google Scholar]
- Han, Ji Won, Pauline Maillard, Danielle Harvey, Evan Fletcher, Oliver Martinez, David K. Johnson, Olichney John M., et al. 2020a. “Vascular Brain Injury and Neurodegeneration Influence Cognition Independent of Amyloid Pathology.” Neurology, in Press. [DOI] [PMC free article] [PubMed]
- Han Ji Won, Maillard Pauline, Harvey Danielle, Fletcher Evan, Martinez Oliver, Johnson David K, Olichney John M. Association of Vascular Brain Injury, Neurodegeneration, Amyloid, and Cognitive Trajectory. Neurology. 2020;95(19):e2622–e2634. doi: 10.1212/WNL.0000000000010531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanseeuw Bernard J, Betensky Rebecca A, Jacobs Heidi IL, Schultz Aaron P, Jorge Sepulcre J., Becker Alex, Orozco Danielle M, Cosio Association of Amyloid and Tau with Cognition in Preclinical Alzheimer Disease: A Longitudinal Study. JAMA Neurology. 2019;76(8):915–924. doi: 10.1001/jamaneurol.2019.1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy John A., Higgins Gerald A. Alzheimer’s Disease: The Amyloid Cascade Hypothesis. Science; Washington. 1992;256(5054):184. doi: 10.1126/science.1566067. https://search.proquest.com/docview/213544666/abstract/1CA079E8ED424ABAPQ/1 [DOI] [PubMed] [Google Scholar]
- Hardy John, Selkoe Dennis J. The Amyloid Hypothesis of Alzheimer’s Disease: Progress and Problems on the Road to Therapeutics. Science. 2002;297(5580):353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hinton Ladson, Carter Kimberly, Reed Bruce R, Beckett Laurel, Lara Esther, DeCarli Charles, Mungas Dan. Recruitment of a Community-Based Cohort for Research on Diversity and Risk of Dementia. Alzheimer Dis. Assoc. Disord. 2010;24(3):234. doi: 10.1097/WAD.0b013e3181c1ee01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikonomovic Milos D., Klunk William E., Abrahamson Eric E., Mathis Chester A., Price Julie C., Tsopelas Nicholas D., Lopresti Brian J. Post-Mortem Correlates of in Vivo PiB-PET Amyloid Imaging in a Typical Case of Alzheimer’s Disease. Brain. 2008;131(6):1630–1645. doi: 10.1093/brain/awn016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack Clifford R, Knopman David S, Jagust William J, Petersen Ronald C, Weiner Michael W, Aisen Paul S, Shaw Leslie M. Tracking Pathophysiological Processes in Alzheimer’s Disease: An Updated Hypothetical Model of Dynamic Biomarkers. The Lancet Neurology. 2013;12(2):207–216. doi: 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson Keith A, Sperling Reisa A, Gidicsin Christopher M, Carmasin Jeremy S, Maye Jacqueline E, Coleman Ralph E, Reiman Eric M. Florbetapir (F18-AV-45) Pet to Assess Amyloid Burden in Alzheimer’s Disease Dementia, Mild Cognitive Impairment, and Normal Aging. Alzheimer’s & Dementia. 2013;9(5):S72–S83. doi: 10.1016/j.jalz.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy William J, Gentle James E. Statistical Computing. Routledge. 2018 [Google Scholar]
- La Joie, Renaud, Adrienne V Visani, Suzanne L Baker, Jesse A Brown, Viktoriya Bourakova, Jungho Cha, Kiran Chaudhary, et al. 2020. “Prospective Longitudinal Atrophy in Alzheimer’s Disease Correlates with the Intensity and Topography of Baseline Tau-Pet.” Science Translational Medicine 12 (524). [DOI] [PMC free article] [PubMed]
- Landau Susan M, Breault Christopher, Joshi Abhinay D, Pontecorvo Michael, Mathis Chester A, Jagust William J, Mintun Mark A. Amyloid-β Imaging with Pittsburgh Compound B and Florbetapir: Comparing Radiotracers and Quantification Methods. J. Nucl. Med. 2013;54(1):70–77. doi: 10.2967/jnumed.112.109009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langa Kenneth M, Burke James F. Preclinical Alzheimer Disease—Early Diagnosis or Overdiagnosis? JAMA Internal Medicine. 2019;179(9):1161–1162. doi: 10.1001/jamainternmed.2019.2629. [DOI] [PubMed] [Google Scholar]
- Leuzy Antoine, Zimmer Eduardo Rigon, Heurling Kerstin, Rosa-Neto Pedro, Gauthier Serge. Use of Amyloid PET Across the Spectrum of Alzheimer’s Disease: Clinical Utility and Associated Ethical Issues. Amyloid. 2014;21(3):143–148. doi: 10.3109/13506129.2014.926267. [DOI] [PubMed] [Google Scholar]
- Marchant Natalie L, Reed Bruce R, Sanossian Nerses, Madison Cindee M, Kriger Stephen, Dhada Roxana, Mack Wendy J. The Aging Brain and Cognition: Contribution of Vascular Injury and aβ to Mild Cognitive Dysfunction. JAMA Neurology. 2013;70(4):488–495. doi: 10.1001/2013.jamaneurol.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus Charles, Mena Esther, Subramaniam Rathan M. Brain PET in the Diagnosis of Alzheimer’s Disease. Clin. Nucl. Med. 2014;39(10):e413–e426. doi: 10.1097/RLU.0000000000000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis Chester A., Bacskai Brian J., Kajdasz Stephen T., McLellan Megan E., Frosch Matthew P., Hyman Bradley T., Holt Daniel P. A Lipophilic Thioflavin-T Derivative for Positron Emission Tomography (PET) Imaging of Amyloid in Brain. Bioorg. Med. Chem. Lett. 2002;12(3):295–298. doi: 10.1016/s0960-894x(01)00734-x. [DOI] [PubMed] [Google Scholar]
- Mathis Chester A, Wang Yanming, Holt Daniel P, Huang Guo-Feng, Debnath Manik L, Klunk William E. Synthesis and Evaluation of 11C-Labeled 6-Substituted 2-Arylbenzothiazoles as Amyloid Imaging Agents. J. Med. Chem. 2003;46(13):2740–2754. doi: 10.1021/jm030026b. [DOI] [PubMed] [Google Scholar]
- Mormino Elizabeth C, Betensky Rebecca A, Hedden Trey, Schultz Aaron P, Amariglio Rebecca E, Rentz Dorene M, Johnson Keith A, Sperling Reisa A. Synergistic Effect of β-Amyloid and Neurodegeneration on Cognitive Decline in Clinically Normal Individuals. JAMA Neurology. 2014;71(11):1379–1385. doi: 10.1001/jamaneurol.2014.2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris Elizabeth, Chalkidou Anastasia, Hammers Alexander, Peacock Janet, Summers Jennifer, Keevil Stephen. Diagnostic Accuracy of 18 F Amyloid Pet Tracers for the Diagnosis of Alzheimer’s Disease: A Systematic Review and Meta-Analysis. Eur. J. Nucl. Med. Mol. Imaging. 2016;43(2):374–385. doi: 10.1007/s00259-015-3228-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris John C, Weintraub Sandra, Chui Helena C, Cummings Jeffrey, DeCarli Charles, Ferris Steven, Foster Norman L. The Uniform Data Set (UDS): Clinical and Cognitive Variables and Descriptive Data from Alzheimer Disease Centers. Alzheimer Dis. Assoc. Disord. 2006;20(4):210–216. doi: 10.1097/01.wad.0000213865.09806.92. [DOI] [PubMed] [Google Scholar]
- Mungas Dan, Reed Bruce R, Crane Paul K, Haan Mary N, González Hector. Spanish and English Neuropsychological Assessment Scales (SENAS): Further Development and Psychometric Characteristics. Psychol. Assess. 2004;16(4):347. doi: 10.1037/1040-3590.16.4.347. [DOI] [PubMed] [Google Scholar]
- Mungas Dan, Reed Bruce R, Farias Sarah Tomaszewski, Decarli Charles. Criterion-Referenced Validity of a Neuropsychological Test Battery: Equivalent Performance in Elderly Hispanics and Non-Hispanic Whites. Journal of the International Neuropsychological Society. 2005;11(5):620–630. doi: 10.1017/S1355617705050745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson Peter T, Alafuzoff Irina, Bigio Eileen H, Bouras Constantin, Braak Heiko, Cairns Nigel J, Castellani Rudolph J. Correlation of Alzheimer Disease Neuropathologic Changes with Cognitive Status: A Review of the Literature. J. Neuropathol. Exp. Neurol. 2012;71(5):362–381. doi: 10.1097/NEN.0b013e31825018f7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovici Gil D, Gatsonis Constantine, Apgar Charles, Chaudhary Kiran, Gareen Ilana, Hanna Lucy, Hendrix James. Association of Amyloid Positron Emission Tomography with Subsequent Change in Clinical Management Among Medicare Beneficiaries with Mild Cognitive Impairment or Dementia. JAMA. 2019;321(13):1286–1294. doi: 10.1001/jama.2019.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinovici Gil D, Hillner Bruce, Whitmer Rachel A, Carrillo Maria, Gatsonis Constantine, Siegel Barry. Imaging Dementia: Evidence for Amyloid Scanning (IDEAS)—a National Study to Evaluate the Clinical Utility of Amyloid Pet. Alzheimer’s & Dementia: The Journal of the Alzheimer’s Association. 2015;11(7):P263–P264. [Google Scholar]
- Rowe Christopher C, Bourgeat Pierrick, Ellis Kathryn A, Brown Belinda, Lim Yen Ying, Mulligan Rachel, Jones Gareth. Predicting Alzheimer Disease with β-Amyloid Imaging: Results from the Australian Imaging, Biomarkers, and Lifestyle Study of Ageing. Ann. Neurol. 2013;74(6):905–913. doi: 10.1002/ana.24040. [DOI] [PubMed] [Google Scholar]
- Dennis J. Selkoe John Hardy The Amyloid Hypothesis of Alzheimer’s Disease at 25 Years EMBO Molecular Medicine 8 6 2016 595 608 https://doi.org/10.15252/emmm.201606210. [DOI] [PMC free article] [PubMed]
- Shea Yat-Fung, Barker Warren, Greig-Gusto Maria T, Loewenstein David A, DeKosky Steven T, Duara Ranjan. Utility of Amyloid Pet Scans in the Evaluation of Patients Presenting with Diverse Cognitive Complaints. Journal of Alzheimer’s Disease. 2018;66(4):1599–1608. doi: 10.3233/JAD-180683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibshirani Robert. Regression Shrinkage and Selection via the Lasso. J. Roy. Stat. Soc.: Ser. B (Methodol.) 1996;58(1):267–288. [Google Scholar]
- Vemuri Prashanthi, Lesnick Timothy G, Przybelski Scott A, Knopman David S, Preboske Greg M, Kantarci Kejal, Raman Mekala R. Vascular and Amyloid Pathologies Are Independent Predictors of Cognitive Decline in Normal Elderly. Brain. 2015;138(3):761–771. doi: 10.1093/brain/awu393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassenko Andrei G., Benzinger Tammie L.S., Morris John C. “PET Amyloid-Beta Imaging in Preclinical Alzheimer’s Disease”. Biochimica et Biophysica Acta (BBA) - Molecular Basis of Disease. Imaging Brain Aging and Neurodegenerative Disease. 2012;1822(3):370–379. doi: 10.1016/j.bbadis.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidman David A, Zamrini Edward, Sabbagh Marwan N, Jacobson Sandra, Burke Anna, Belden Christine, Powell Jessica. Added Value and Limitations of Amyloid-Pet Imaging: Review and Analysis of Selected Cases of Mild Cognitive Impairment and Dementia. Neurocase. 2017;23(1):41–51. doi: 10.1080/13554794.2017.1290806. [DOI] [PubMed] [Google Scholar]
- Weintraub Sandra, Salmon David, Mercaldo Nathaniel, Ferris Steven, Graff-Radford Neill R, Chui Helena, Cummings Jeffrey. The Alzheimer’s Disease Centers’ Uniform Data Set (UDS): The Neuropsychological Test Battery. Alzheimer Dis. Assoc. Disord. 2009;23(2):91. doi: 10.1097/WAD.0b013e318191c7dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.