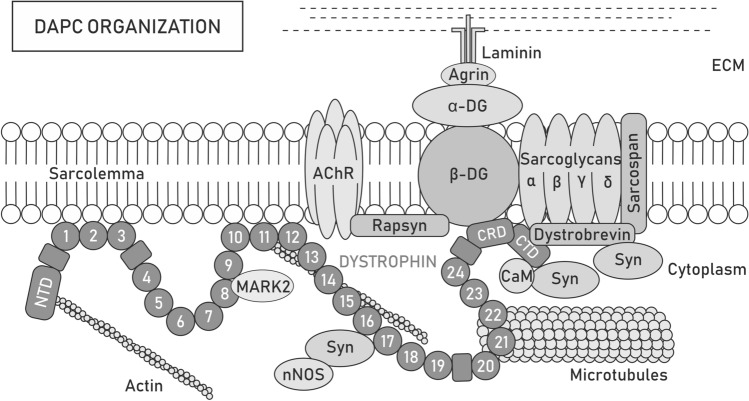
Fig. 1.
Dystrophin-associated protein complex organization. Dystrophin forms a scaffold for the dystrophin-associated protein complex (DAPC) that acts both as a mechanical force transducer and a signaling hub. Depending on the presence of the dystrophin isoform, its paralog utrophin or tissue/cellular localization, the content of DAPC may differ. Full-length dystrophin Dp427 consists of the N-terminal domain (NTD), central rod [with 24 spectrin-like repeats (circles) and 4 hinge modules (rectangles)], cysteine-rich (CRD), and C-terminal (CTD) domains, which provide multiple sites for interactions with proteins. NTD and spectrin-like repeats 11–15 bind costameric actin filaments, while repeats 8 and 9 anchor MARK2 and 20–23 interact with microtubules, which can also contact dystrophin indirectly through ankyrin-B. Spectrin-like repeats 1–3 and 10–12 may additionally participate in stabilizing the complex to the sarcolemma by binding the lipid bilayer. Through CRD, dystrophin interacts with sarcolemma-located β-dystroglycan (β-DG), which is anchored to α-dystroglycan (α-DG). In turn, α-DG binds laminin, a component of the extracellular matrix (ECM) network, which facilitates the transfer of forces during muscle contraction from the cytoskeleton to the ECM and protects the sarcolemma from twich-induced damage. At the neuromuscular junction (NMJ), the interaction of α-DG with agrin and perlecan provides MuSK-induced clustering of acetylcholine receptors (AChRs) and determines the localization of acetylcholine esterase, while the binding of β-DG with rapsyn is involved in the clustering of AChRs. Additionally, β-DG stabilizes α, β, δ- and γ-sarcoglycan and the sarcospan complex at the sarcolemma. The CTD of dystrophin interacts with various cytosolic proteins, such as dystrobrevin or syntrophins (Syn). Syntrophins recruit sodium channels and signaling molecules, such as neuronal nitric oxide synthase (nNOS)