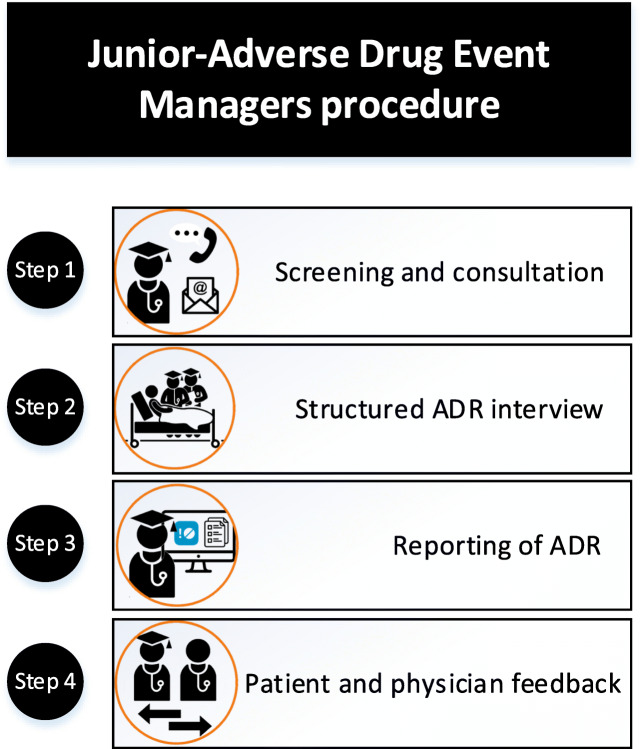
Fig. 1.
Junior-Adverse Drug Event Managers procedure. The first step consisted of identifying all patients with potential ADRs by screening or being consulted by a healthcare professional. The second step consisted of reviewing the patient’s electronic patient record (EPR) and performing a thorough medication and side effect interview with the patient. The third step consisted of reporting the ADR to the Netherlands Pharmacovigilance Center Lareb and handling all follow-up questions. The final step consisted of providing the attending physician with feedback received from Lareb and uploading this information into the patient’s EPR