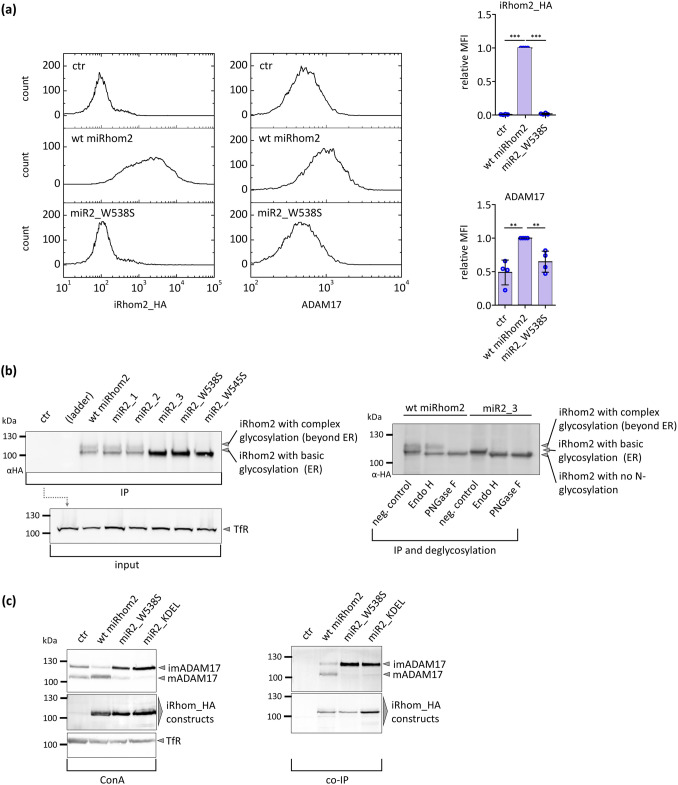
Fig. 4.
The integrity of the conserved motif within the IRHD is crucial for ER to Golgi transport. HEK293 cells stably expressing the indicated iRhom constructs or GFP (ctr) were utilised. a Cell surface expression of iRhom constructs and endogenous ADAM17 were measured by flow cytometry. For quantification, the geometric mean of the fluorescence intensity was normalised to the wt miRhom2 sample. n = 4. b Due to glycosylation murine iRhom2 is detectable as two separate bands by western blotting. Left panel: Different iRhom constructs were immunoprecipitated (IP) and immunoblotted. Right panel: Deglycosylation experiments were performed with immunoprecipitations of wt miRhom2 and miR2_3, and analysed by immunoblotting. IP samples were treated with either Endo H or PNGase F. As negative control samples were left untreated. The results show that the upper band of wt_iRhom2 is resistant to the treatment with endo H while the lower band slightly shifts to a lower molecular weight, which is the fraction without N-glycosylation. Treatment with PNGase F shifts the upper band also to the molecular weight of the fraction without N-glycosylation. n = 3. c To study the maturation of ADAM17, glycosylated proteins were enriched by concanavalin A beads (ConA). To specifically analyse the binding between ADAM17 and iRhom constructs, co-immunoprecipitations (co-IP) were performed by using the iRhom constructs (tagged with HA tag) as bait. Maturation and binding were assessed by immunoblotting. The transferrin receptor (TfR) served as input control. ADAM17 maturation was evaluated by comparing the amount of immature (imADAM17) and the amount of mature ADAM17 (mADAM17). Quantitative analysis of ADAM17 maturation levels and ADAM17 binding can be found in figures S7c, d. n > 3