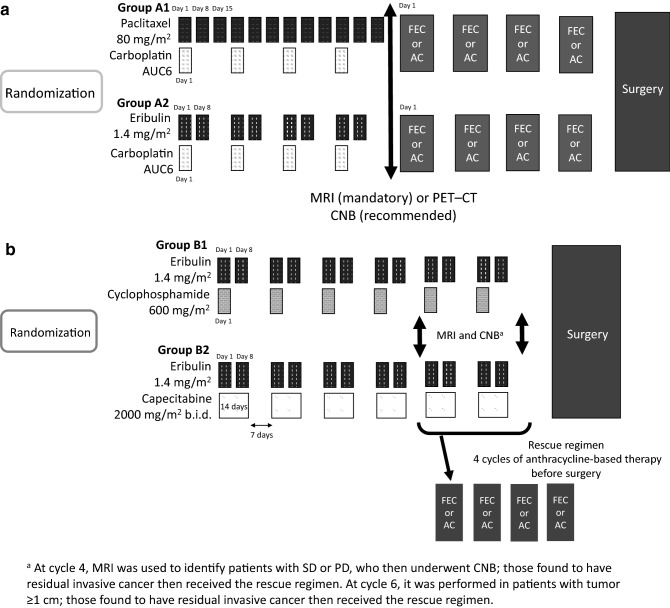
Fig. 2.
Dosing schedule. a Patients in group A1 received 4 cycles of combination therapy with paclitaxel (80 mg/m2) on days 1, 8, and 15 of each cycle and carboplatin (AUC6) on day 1. Patients in group A2 received 4 cycles of combination therapy with eribulin (1.4 mg/m2) on days 1 and 8 of each cycle and carboplatin (AUC6) on day 1. Depending on the antitumor effects of the initial therapy, evaluated at cycle 4, patients subsequently received the anthracycline-based regimen before surgery, or discontinued chemotherapy and underwent surgery. The anthracycline-based regimen comprised FEC, consisting of 5-fluorouracil (500 mg/m2), epirubicin (100 mg/m2), and cyclophosphamide (500 mg/m2); or AC, consisting of doxorubicin (60 mg/m2) and cyclophosphamide (600 mg/m2), on day 1 of each cycle. b Patients in group B1 received 4 cycles of eribulin (1.4 mg/m2) on days 1 and 8 of each cycle and cyclophosphamide (600 mg/m2) on day 1. Patients in group B2 received 4 cycles of eribulin (1.4 mg/m2) on days 1 and 8 of each cycle and capecitabine (2000 mg/m2/day) administered orally twice daily on days 1–14. Depending on the antitumor effects, assessed at cycle 4, patients in groups B1 and B2 received an additional 2 cycles of the eribulin-based regimen before surgery, or underwent surgery after receiving the rescue regimen (FEC or AC as described for group A). AC, doxorubicin–cyclophosphamide regimen; CNB, core needle biopsy; CT, computed tomography; FEC, 5-fluorouracil–epirubicin–cyclophosphamide regimen; MRI, magnetic resonance imaging; PD, progressive disease; PET, positron emission tomography; SD, stable disease