Fig. 1.
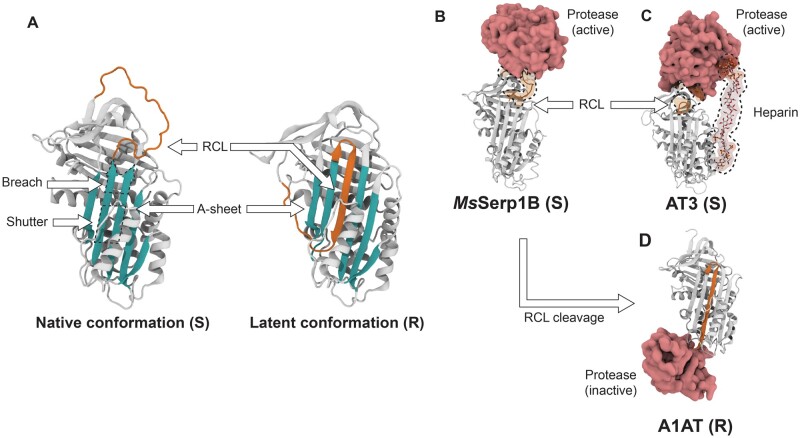
Serpin fold and mechanistic diversity. (S) and (R) denote stressed and relaxed conformations, respectively. (A) Cartoon representation of the S to R state conformational transition, in alpha-1-antitrypsin (A1AT). The RCL in both native (exposed) and latent (inserted) conformations is highlighted, among other key structural components of the serpin fold, such as the breach, shutter, and A-sheet (PDBs: native stressed conformation 3NE4, latent relaxed conformation 1IZ2). (B) Noncovalent Michaelis complex between Manduca sexta serpin1B (MsSerp1B) and active trypsin protease (PDB: 1K9O). (C) Ternary michaelis complex between human antithrombin III (AT3) with heparin templated active thrombin (PDB: 1TB6). (D) Covalent complex between cleaved A1AT and inhibited trypsin, post-RCL cleavage (PDB: 1EZX).