Abstract
Background
This study investigated the efficacy and safety of providing medication for opioid use disorder (MOUD) and individualized telehealth in Kentucky, a state severely impacted simultaneously by the opioid epidemic and the COVID-19 pandemic.
Methods
The investigation analyzed pre- and post-COVID-19 characteristics in 191 opioid use disorder (OUD) buprenorphine outpatients who completed an 18-question survey in late 2020 related to COVID testing, OUD relapses, obstacles to maintaining abstinence, and treatment resources.
Results
The study revealed no statistically significant changes in drug use before and after the onset of the COVID-19 pandemic despite monthly volume increases. Results further demonstrated statistically significant barriers to treatment, including loss of housing and transportation, food insecurity, and onset of depression. No patients required hospitalization or succumbed to OUD or COVID-19. Potentially effective resource utilization findings included clinic transportation and 24/7 crisis intervention. Respondents rated telehealth as helpful when used in an individualized hybrid model matching patient's need to available resources based on COVID-19 safety guidelines.
Conclusion
This report yields key clinical insights into providing outpatient MOUD care during the COVID-19 pandemic, validating in-person care as both safe and effective. Patients' experiences proved helpful in identifying and quantifying obstacles to abstinence in conjunction with facilitating continued patient access to essential clinical resources. Notably, telehealth can supplement rather than replace in-person treatment.
Keywords: Opioid use disorder (OUD), Medication for opioid use disorder (MOUD), COVID-19, Buprenorphine, Telehealth, Addiction, Treatment
1. Introduction
Since the onset of the opioid epidemic two decades ago, half a million Americans have perished from opioid overdose (Wen & Sadeghi, 2020; Wilson et al., 2020). In the first 12 months of the COVID-19 pandemic (March 2020 through February 2021), the Centers for Disease Control and Prevention (CDC, COVID Data Tracker, 2021) reported a similar number succumbed to the coronavirus in a fraction of the time.
Alexander et al. (2020), one of the first in the medical literature to call attention to the vulnerability of patients with opioid use disorder (OUD), succinctly characterized the situation as “an epidemic in the midst of a pandemic.” In the same issue, Becker and Fiellin (2020) and Volkow (2020) echo similar anxieties and present their own concerns regarding looming challenges. Khatri and Perrone (2020) list numerous barriers to care exacerbated by the pandemic, while Stratton (2020) discusses global challenges to emergency medical services (EMS) operations.
Mellis et al. (2021) studied post-COVID-19 responses from both single- and multi-substance users via a nationwide survey of patients identified by the Addiction Policy Forum, concluding that the latter were more likely to report problems with access to treatment and services and more likely to use telehealth. Samuels et al. (2020) described the Rhode Island buprenorphine hotline that functions as a 24/7 audio-only “tele-bridge” for people with moderate to severe OUD to be linked to a qualified provider for initial assessment and unobserved induction followed by real-time linkage to ongoing outpatient maintenance treatment.
Considering the paucity of clinical data published to date, this study reports firsthand experience in addressing previously identified obstacles to accessing treatment for OUD, including medications for opioid use disorder (MOUD) and leveraging potential opportunities in a clinical setting.
2. Materials and methods
The research team conducted this study in Louisville at the largest facility in a network of office-based multi-specialty outpatient addiction clinics in Kentucky, a state hit particularly hard by the opioid epidemic (CDC, Health Advisory Web Site, 2020). In-house providers specialize in addiction, psychiatry, primary care, and opioid-free pain management. Patients with OUD receive buprenorphine treatment in conjunction with mandatory drug testing, individual counseling, and group counseling along with other addiction-related programs, all coordinated by case managers. Due in part to Kentucky's mandate that Medicaid provide behavioral care benefits, 99% of clinic patients possess insurance coverage, including Medicaid (79%), Medicare (6%), and commercial (14%). The remaining 1% represent a small pool of temporarily uninsured patients, most of whom are waiting for Medicaid approval or eligibility from commercial insurance when changing carriers. The network provides a broad spectrum of in-house resources in addition to individual and group counseling. Examples of these resources include definitive GC/MS urine testing and both immunoassay and PCR COVID-19 testing, primary care (limited to the clinics' substance use disorder [SUD] patient roster), free clinic van transportation, case management, social services, job placement, DUI compliance, and court coordination. More recently, the clinics added in-house psychiatry and opioid-free pain management.
From the onset of the COVID-19 pandemic, all sites implemented CDC-compliant adaptations to facilitate continued in-person provision of MOUD and multidisciplinary care. Additionally, starting in mid-March 2020 when Kentucky implemented new COVID-19 restrictions statewide, the network immediately expanded previously limited Zoom telehealth capability. Participation remained optional but available to all patients.
To elicit direct feedback from patients who struggle to achieve and maintain abstinence, while simultaneously confronting the socioeconomic challenges of COVID-19, one site conducted a patient survey on the barriers contributing to relapse along with potential solutions to the epidemic within the pandemic. Investigators recorded responses from a convenience sample of 191 patients (approximately one-third of total active clinic patients in the Louisville MOUD program) seen by providers on two nonconsecutive days in the first week of November. The questionnaire comprised 18 questions related to multiple topics comparing care before and after the onset of the pandemic, including COVID-19 testing, abstinence (defined as all illicit substances per standing clinic policy), potential obstacles to maintaining abstinence (loss of employment, housing, or transportation, onset of depression, food insecurity, difficulty maintaining abstinence), available clinical treatment resources (busing, 24/7 crisis counseling, other clinical services), and patient suggestions. The team formulated questions a priori to facilitate eventual classification of respondents into four groups representing patient outcomes with respect to drug use versus drug nonuse before and after COVID-19.
Trained triage staff queried all patients in person during their visit regarding their experience during the 12 months prior to recognition of the pandemic in March 2020 versus thereafter through October 2020. The staff administered all interviews using a single-page questionnaire as a script with staff documenting patients' responses on the same form in real time. Staff assured all participants that their responses would be taken under consideration to improve overall care as appropriate. Abstractors later retrieved additional data not included in the questionnaire regarding demographics, insurance coverage, and telehealth utilization. The authors then entered the data into a Microsoft Excel spreadsheet for statistical analysis utilizing ANOVA for parametric data and Chi-square for non-parametric data using a secure computer located in the clinic. The Institutional Review Board at the University of Louisville approved the investigation, including the clinical chart and patient survey components.
3. Results
3.1. Patient characteristics
Patients' ages ranged from 21 to 73 years old (mean 38). Table 1 illustrates questionnaire findings by gender and race. The sample included 95 males (49.7%); 96 females (50.3%). The sample included 186 whites (97.5%), two Blacks (1.0%), one Hispanic (0.5%), one other (0.5%), and one declined to answer (0.5%). Insurance coverage was mostly Medicaid (79%), followed by 14% commercial, 6% Medicare, and 1% uninsured.
Table 1.
Patient race/ethnicity using CMSa nomenclature.
| Male | Female | Total | |
|---|---|---|---|
| White | 92 (49.5%) | 94 (50.5%) | 186 (97.5%) |
| Black | 1 (0.55) | 1 (0.5%) | 2 (1.0%) |
| Hispanic | 0 (0%) | 1 (0.5%) | 1 (0.5%) |
| Other | 1 (0.5%) | 0 (0%) | 1 (0.5%) |
| Declined | 1 (0.5%) | 0 (0%) | 1 (0.5%) |
| Total | 95 (49.7%) | 96 (50.3%) | 191 |
Centers for Medicare & Medicaid Services.
Monthly volume for active patients increased throughout 2020. January totaled 404 active patients rising to 481 in February. Starting with the onset of COVID-19, patient volume rose during each of the following months from 535 in March to 633 in October (the highest of any month), averaging 577 patients for the eight-month post-COVID time frame. Survey respondents included 191 of these patients as noted above (Methodology). Length of treatment (patient retention) ranged from one month to three years, with the latter approximating initial opening of the clinic. Overall, 145 of 191 (75.9%) participants logged one year or less, 34 (17.8%) logged one to two years, and 12 (6.3%) logged two to three years.
No subjects developed COVID-19 disease requiring hospitalization. No active patients expired from COVID-19 or from substance use–related causes during the coronavirus phase of the study (March through October 2020). One inactive patient, who had dropped out of the clinic several weeks earlier, died of an opioid overdose in 2020.
The investigators divided patients into four groups (Table 2 ) based on drug use versus drug nonuse before and after the start of the COVID-19 pandemic. Fig. 1 illustrates the relative number of patients a) not using before or using after, b) using before but not using after, c) not using before but using after, and d) using before and using after.
Table 2.
Patient responses grouped by drug use before and after onset of COVID-19.
| Non-using before Non-using after (N = 104) |
Using before Non-using after (N = 15) |
Non-using before Using after (N = 17) |
Using before Using after (N = 55) |
Total (N = 191) |
p value* | |
|---|---|---|---|---|---|---|
| Age | ||||||
| Mean (standard deviation) | 39.7 (10.8) | 37.1 (9.7) | 37.2 (11) | 36.1 (9.5) | 38.2 (10.4) | NS |
| COVID testing | ||||||
| Tested (%) | 68 (65.4) | 8 (53.3) | 11 (64.7) | 41 (74.5) | 128 (67) | NS |
| Positive (%) | 2 (2.9) | 0 (0) | 0 (0) | 3 (7.3) | 5 (3.9) | NS |
| Obstacles encountered | ||||||
| Loss of employment (%) | 33 (31.7) | 7 (46.7) | 6 (35.3) | 22 (40.0) | 68 (35.6) | NS |
| Onset of depression (%) | 28 (26.9) | 3 (20.0) | 13(76.5) | 25 (45.5) | 69 (36.1) | <0.001 |
| Loss of transportation (%) | 7 (6.7) | 0 (0) | 3 (17.6) | 9 (16.4) | 19 (9.9) | NS |
| Insecurity of food (%) | 10 (9.6) | 0 (0) | 7 (41.2) | 16 (29.1) | 33 (17.3) | <0.001 |
| Loss of housing (%) | 1 (1.0) | 1 (6.7) | 2 (11.8) | 7 (12.7) | 11 (5.8) | 0.015 |
| Difficulty maintaining abstinence (%) | 7 (6.7) | 2 (13.3) | 12 (70.6) | 29 (52.7) | 50 (26.2) | <0.001 |
| Reliance on clinical resources | ||||||
| Clinic busing (%) | 19 (18.3) | 2 (13.3) | 7 (41.2) | 21 (38.2) | 49 (25.7) | 0.013 |
| 24/7 crisis counseling (%) | 29 (27.9) | 3 (20.0) | 3 (17.6) | 28 (50.9) | 63 (33.0) | 0.007 |
| Other clinical support (%) | 61 (58.7) | 11 (73.3) | 14 (82.4) | 45 (81.8) | 131 (68.6) | NS |
NS: not significant (significance requires p-value <0.05).
Fig. 1.
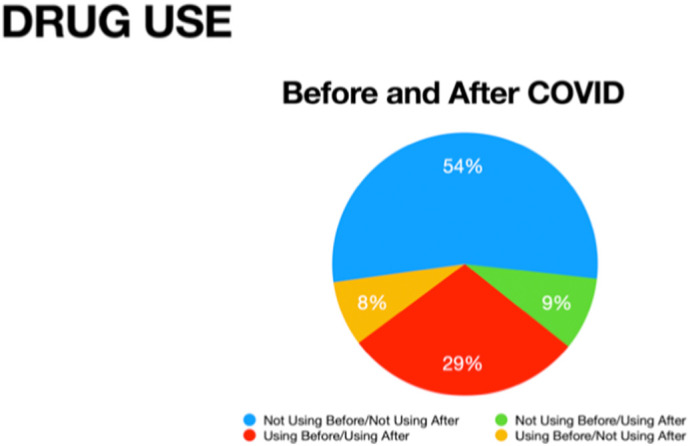
Drug use in patients before and after COVID-19.
No statistically significant changes (p < 0.05) were detected before vs. after.
3.2. Clinical treatment
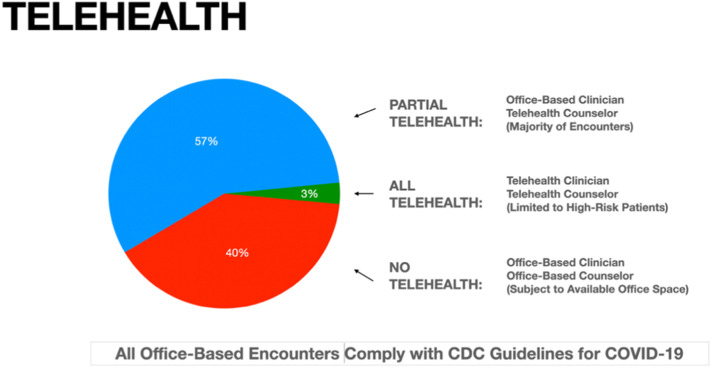
Although the clinic offered telehealth to all patients for all encounters, results showed a marked difference in utilization between medical and counseling services following onset of the pandemic (Fig. 2 ). Encounters with clinical providers remained essentially unchanged with only 18 patients (3.1%) of the average monthly volume of 577 patients switching to telehealth due to high-risk medical co-morbidities and/or COVID-19 positivity. Due to their medical fragility and risk of coronavirus transmission, all 18 high-risk patients also received both individual and group counseling via telehealth for the duration of the study.
Fig. 2.
Use of telehealth during COVID-19 by clinicians and counselors.
All but high-risk patients (3%) continued in-person clinical care.
The majority of patients (57%) migrated to telehealth counseling due to space limitations necessitated by COVID-19 safety precautions.
In contrast, the clinics provided the majority of counseling services to 327 patients (56.7%) during the pandemic via telehealth due to limited availability of CDC-compliant individual and group counseling space. The remaining 232 patients (40.2%) continued in-person group counseling (reduced from 12 to 6 patients) and individual counseling using standard COVID-19 safety precautions: face masks, social distancing, and hand hygiene. All patients continued in-person drug testing for every encounter, including the high-risk patients otherwise using telehealth exclusively.
3.3. Survey data on obstacles and resources
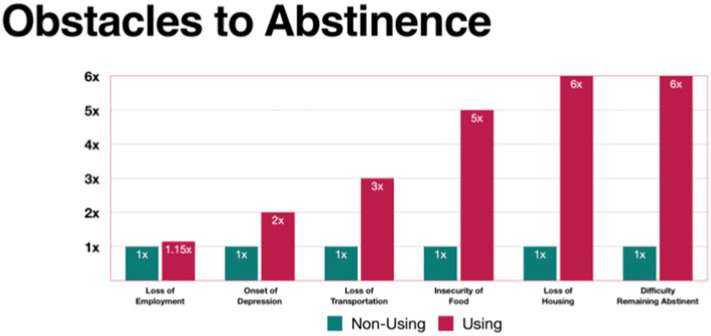
Obstacles to abstinence included loss of employment, onset of depression, loss of transportation, food insecurity, loss of housing, and difficulty maintaining abstinence. Fig. 3 illustrates each in terms of likelihood of occurrence for relapsed versus nonusing patients. Four of the six categories showed statistically significant correlation with relapse; only loss of employment and loss of transportation did not demonstrate significance (Table 2).
Fig. 3.
Likelihood of obstacles reported as contributing to relapse.
(Values for non-using patients are set to 1× to facilitate comparisons with using patients.)
All but loss of employment and loss of transportation were statistically significant (p < 0.05).
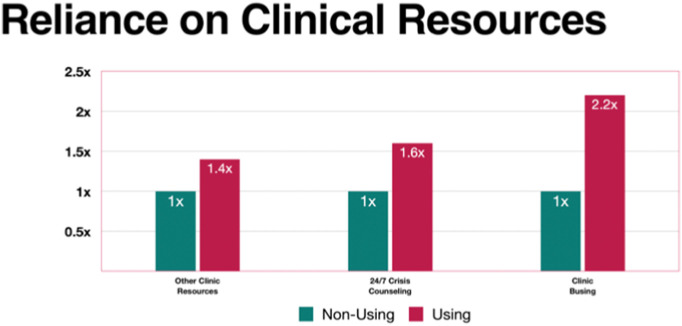
Resources rated as important included reliance on clinic busing, clinic 24/7 crisis counseling, and other clinic services. Fig. 4 illustrates each in terms of likelihood of occurrence for relapsed versus nonusing patients. Two of the three categories showed statistically significant correlation with relapse; only “other clinic resources” did not demonstrate such significance (Table 2).
Fig. 4.
Likelihood of reliance on clinical resources to avoid relapse.
(Values for non-using patients are set to 1× to facilitate comparisons with using patients.)
All but other clinic resources were statistically significant (p < 0.05).
4. Discussion
This investigation reports one of the first analyses of providing comprehensive, ongoing clinical care for OUD patients using buprenorphine (MOUD) in an office-based outpatient treatment (OBOT) setting both before and after the onset of the COVID-19 pandemic. Early in the COVID-19 pandemic, multiple authors (Alexander et al., 2020; Becker & Fiellin, 2020; Khatri & Perrone, 2020; Stratton, 2020; Volkow, 2020) expressed concerns about the potential for adverse interactions with the opioid epidemic. The current study reveals the reality of these potential concerns that OUD patients faced. Survey data confirmed certain obstacles to abstinence, including those that correlated with relapse, providing new insight into areas for potential fund allocation. The clinical sites found a low rate of COVID-19 incidence despite continuing in-person services for those at low risk of COVID-19 complications.
Three expert panels have articulated concerns and proposed solutions to the epidemic within the pandemic (Jemberie et al., 2020; López-Pelayo et al., 2020; Ornell et al., 2020). Bojdani et al. (2020) offered a comprehensive perspective, providing a systematic review of 13 articles addressing the impact of the COVID-19 pandemic on psychiatric care in the United States. Recognizing that the current study revealed 69 (36.1%) individuals with new onset of depression, Bodjani's observations and recommendations appear particularly relevant to OUD patients who often suffer from one or more psychiatric co-morbidities.
Huskamp et al. (2020) used commercial and Medicare Advantage insurance claims data for OUD treatment, including medication fills, outpatient visits, and urine tests among insured individuals compared to the previous year (2019), and found no decrease in medication fills or clinician visits. Fewer new patients initiated OUD medications, however, and less urine testing occurred across all patients. The current study found an increase of patient visits as the pandemic progressed, indicating individuals maintained access to clinicians and adequate treatment (including MOUD). With the facility and comprehensive support services remaining open, the OUD patient roster described in this study increased from 535 to 628. Despite the pandemic, investigators opened another clinic serving 150 new patients in Campbellsville, a predominantly rural area 90 miles south of Louisville in central Kentucky. (The current study does not include any Campbellsville patients.)
Not surprisingly, patients who relapsed reported challenges with each of the surveyed obstacles (housing, nutrition, transportation, employment, and depression) at statistically significant frequencies, ranging from two to six times, compared to their nonusing counterparts (Fig. 3), confirming the concerns of numerous authors cited here. Additionally, patients who reported relapse relied on clinical resources at frequencies from 1.4 to 2.2 times those of their nonusing counterparts (Fig. 4). Although the “Other Clinic Resources” category failed to achieve statistical significance, many of the respondents identified reliance on specific resources in this category (discussed here) as essential to their care.
The least anticipated yet possibly most important findings from these data relate to utilization of telehealth (Fig. 2). Although the clinics previously accommodated one-on-one medical provider services, during the pandemic the leadership reconsidered the logistics of counseling services based on space limitations imposed by social distancing. Group counseling continued in smaller (half-size) groups to accommodate necessary distancing. Due to office space limitations, the clinics converted individual counseling encounters (as opposed to clinical provider encounters) to telehealth for the majority (57%) of patients, including monthly drug assessment and counseling services. Despite these changes, the remaining minority (approximately 40%) continued in-person counseling as before. All clinic patients, independent of telehealth classification, continued in-person drug testing for each clinical encounter for the duration of the study based on such testing proving to be indispensable to maintaining accountability and minimizing diversion. This hybrid approach integrates one-on-one meetings with telehealth services based on patients' eligibility and available space, providing a mutually acceptable solution without adverse effects on participation or outcomes.
In their commentary on suggested emergency mitigation of federal and state barriers to OUD treatment, Davis and Samuels (2020) recommended waiving restrictions on a) take-home methadone, b) in-person prescribing for new buprenorphine patients, c) patients incarcerated for low-level crimes, d) patient caps for buprenorphine prescribers, e) naloxone prescriptions, and f) syringe access. Cance and Doyle (2020) studied buprenorphine prescribing data from the Texas prescription monitoring program (PMP) that tracks all controlled substances and found an increase in the number of patients receiving outpatient prescriptions despite a decline in existing patients. The author concluded that the relaxation of federal regulations and positive changes in buprenorphine dispensing adds support for policy-makers to re-evaluate whether these changes should remain temporary.
Researchers in this investigation sought a better understanding of how many OUD patients tested positive for COVID while remaining open. Although the treatment facility remained open and followed guidelines to continue to provide MOUD to qualifying patients, other patients with OUD may have had difficulties gaining comparable access to MOUD, and these difficulties may have been exacerbated by COVID-19. Volkow (2020) likewise surmised that individuals with OUD may be more susceptible to adverse outcomes of COVID-19 due to compromised lung function that may further complicate access to health care. In the eight months after the onset of the pandemic, only 10/577 (1.7%) clinic patients in this investigation tested positive for COVID-19, half of which (five patients) participated in the survey. Of the five, two remained nonusing both before and after the pandemic onset while the other two were using both before and after. These figures, which are at or below both state and local averages, suggest that the policy of strict compliance with CDC guidelines justifies the decision to remain open without curtailing services.
This study has several limitations. The data come from the largest clinic site in a network of outpatient buprenorphine treatment centers but include only OUD patients, limiting generalizability to other populations. Analysts did not evaluate the contribution of polysubstance disorder, which amplifies many of the challenges in SUD treatment. State and local incidence of COVID-19 was relatively low; providers in areas with higher incidence may not choose to undertake similar in-person treatment. The survey attempted to quantify the importance of resources, but qualitative results could yield new insights. This study has a degree of survivorship bias: despite including subjects who did not achieve or maintain abstinence, researchers could not obtain survey data from patients who left the program. These methods could allow for selection bias, by administering surveys on two days in the same week. All subjects in this sample were taking buprenorphine for OUD, limiting applicability to patients who utilize other means of OUD treatment. Finally, this design suffers from social desirability in survey research as individuals may have responded to the survey to please the study investigators. Authors on this project include the chief executive officer (CEO) and chief medical officer (CMO) of the treatment center. Despite these limitations, this study provides empirical data on treatment characteristics and patient feedback related to an urgent problem related to the COVID-19 pandemic.
5. Conclusion
The current study describes clinical experience with treating OUD patients during the COVID-19 pandemic utilizing MOUD with buprenorphine. Rather than restricting OUD treatment during the COVID-19 pandemic, the authors suggest judicious utilization of CDC-compliant safety measures to allow expansion of outpatient MOUD services while maintaining successful outcomes. Opportunities for improving care include mitigating identified barriers (nutrition, housing, transportation, depression) and leveraging potential resources (clinic transportation, 24/7 crisis intervention). Telehealth, particularly when used selectively within a hybrid model matching patients' need to available resources, may prove especially helpful in maintaining compliance with CDC guidelines.
The authors further recommend continued investigation into the intersection of the COVID-19 pandemic and the opioid epidemic, including scrutiny of obstacles for at-risk populations, such as the homeless and those in drug court proceedings.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
CRediT authorship contribution statement
Richard H. Cales: Conceptualization, Methodology, Investigation, Original draft, Review, Editing.
Shannon C. Cales: Conceptualization, Methodology, Investigation, Review.
Jacob Shreffler: Review, Editing, Statistics.
Martin R. Huecker: Review, Editing.
Declaration of competing interest
Richard H. Cales: None (no interest in NuLease Medical Solutions, LLC)
Shannon C. Cales: Sole Manager/Owner, NuLease Medical Solutions, LLC
Jacob Shreffler: None
Martin R. Huecker: None.
Acknowledgments
The authors would like to acknowledge the following for their assistance: Alyssa Thomas for IRB protocols and APA compliance (University of Louisville, Louisville, KY); Campbell Bishop, Jade Barnes, Symone Hartman and Kim Helton for collecting and correlating patient data (NuLease Medical Solutions, LLC, Louisville, KY), and Patti Green for final editing (Providence St. Joseph Health, Portland, OR).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jsat.2021.108543.
Appendix A. Supplementary data
Supplementary material
References
- Alexander G.C., Stoller K.B., Haffajee R.L., Saloner B. An epidemic in the midst of a pandemic: opioid use disorder and COVID-19. Annals of Internal Medicine. 2020;173(1):57–58. doi: 10.7326/M20-1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker W.C., Fiellin D.A. When epidemics collide: coronavirus disease 2019 (COVID-19) and the opioid crisis. Annals of Internal Medicine. 2020;173(1):59–60. doi: 10.7326/M20-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bojdani E., Rajagopalan A., Chen A., Gearin P., Olcott W., Shankar V.…DeLisi L.E. COVID-19 pandemic: impact on psychiatric care in the United States. Psychiatry Research. 2020;289 doi: 10.1016/j.psychres.2020.113069. (published online ahead of print, 2020 May 6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cance J.D., Doyle E. Changes in outpatient buprenorphine dispensing during the COVID-19 pandemic. Journal of the American Medical Association. 2020;324(23):2442–2444. doi: 10.1001/jama.2020.22154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control cdc.gov; 2020. Increase in fatal drug overdoses across the United States driven by synthetic opioids before and during the COVID-19 pandemic. https://emergency.cdc.gov/han/2020/han00438.asp Official Health Advisory web site. (Updated December 17, 2020, 8:00 AM ET, 2021)
- Centers for Disease Control and Prevention COVID Data Tracker. 2021. https://covid.cdc.gov/covid-data-tracker/#datatracker-home
- Davis C.S., Samuels E.A. Opioid policy changes during the COVID-19 pandemic - and beyond. Journal of Addiction Medicine. 2020;14(4):e4–e5. doi: 10.1097/ADM.0000000000000679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huskamp H.A., Busch A.B., Uscher-Pines L., Barnett M.L., Riedel L., Mehrotra A. Treatment of opioid use disorder among commercially insured patients in the context of the COVID-19 pandemic. Journal of the American Medical Association. 2020;324(23):2440–2442. doi: 10.1001/jama.2020.21512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemberie W.B., Williams J.S., Eriksson M., Gronlund A.S., Ng N., Nilsson M.B.…Lundren L.M. Substance use disorders and COVID-19: multi-faceted problems which require multi-pronged solutions. Frontiers in Psychiatry. 2020;11:714. doi: 10.3389/fpsyt.2020.00714. (Jul 21) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatri U.G., Perrone J. Opioid use disorder and COVID-19: crashing of the crises. Journal of Addiction Medicine. 2020;14(4):e6–e7. doi: 10.1097/ADM.0000000000000684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Pelayo H., Aubin H.J., Drummond C., Dorn G., Pascual F., Rehm J.…Gual A. “The post-COVID era”: challenges in the treatment of substance use disorder (SUD) after the pandemic. BMC Medicine. 2020;18(1):241. doi: 10.1186/s12916-020-01693-9. (Jul 31) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellis A.M., Potenza M.N., Hulsey J.N. COVID-19-related treatment service disruptions among people with single- and polysubstance use concerns. Journal of Substance Abuse Treatment. 2021;121 doi: 10.1016/j.jsat.2020.108180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ornell F., Moura H.F., Scherer J.N., Pechansky F., Kessler F.H.P., von Diemen L. The COVID-19 pandemic and its impact on substance use: implications for prevention and treatment. Psychiatry Research. 2020;289 doi: 10.1016/j.psychres.2020.113096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels E.A., Clark S.A., Wunsch C., Jordison Keeler L.A., Reddy N., Vanjani R., Wightman R.S. Innovation during COVID-19: improving addiction treatment access. Journal of Addiction Medicine. 2020;14(4):e8–e9. doi: 10.1097/ADM.0000000000000685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stratton S.J. COVID-19: not a simple public health emergency. Prehospital and Disaster Medicine. 2020;35(2):119. doi: 10.1017/S1049023X2000031X. [DOI] [PubMed] [Google Scholar]
- Volkow N.D. Collision of the COVID-19 and addiction epidemics. Annals of Internal Medicine. 2020;173(1):61–62. doi: 10.7326/M20-1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen L.S., Sadeghi N.B. The opioid crisis and the 2020 US election: crossroads for a national epidemic. Lancet. 2020;396(1):1316–1318. doi: 10.1016/S0140-6736(20)32113-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson N., Kariisa M., Seth P., Smith H., IV, Davis N.L. Drug and opioid-involved overdose deaths—United States, 2017–2018. MMWR. Morbidity and Mortality Weekly Report. 2020;69:290–297. doi: 10.15585/mmwr.mm6911a4externalicon. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material