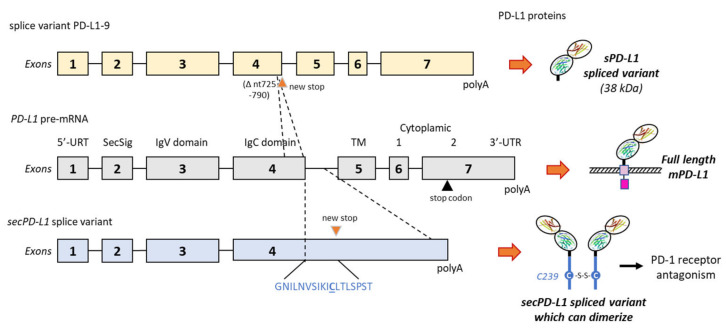
Figure 5.
Two examples of alternative splicing of the PD-L1 pre-mRNA. The exons’ and introns’ organization of full-length PD-L1 is shown in the middle, and the full-length PD-L1 protein is shown on the right with its different domains (as in Figure 1). Above, the splice variant PD-L1-9, which has lost a 66-bp region from nt-725 to 790 in exon 4. The deletion indicates a frame shift leading to a stop codon before the transmembrane domain (TM). The variant produces a truncated sPD-L1 protein (38 kDa) lacking the TM and intracellular domains [48]. Below is an alternatively spliced form of the human PD-L1 cDNA from placental tissue. The variant contains the first 4 exons of PD-L1, including the secretory signal (SecSig) at the N-terminus, IgV and IgC domains, which are shared with the full-length PD-L1. The variant does not splice into the fifth exon (encoding the transmembrane domain) but reads into the fourth intron, within a new stop codon. It produces an mRNA that lacks a transmembrane domain at its 3′ end and leads to a protein with the indicated unique C-terminal sequence. The underlined cysteine residue (C239) allows for protein homodimerization, as represented on the right side. The expressed protein, naturally dimerizing, was found to inhibit T-cell proliferation and production of IFN-γ from activated T-cells [105].