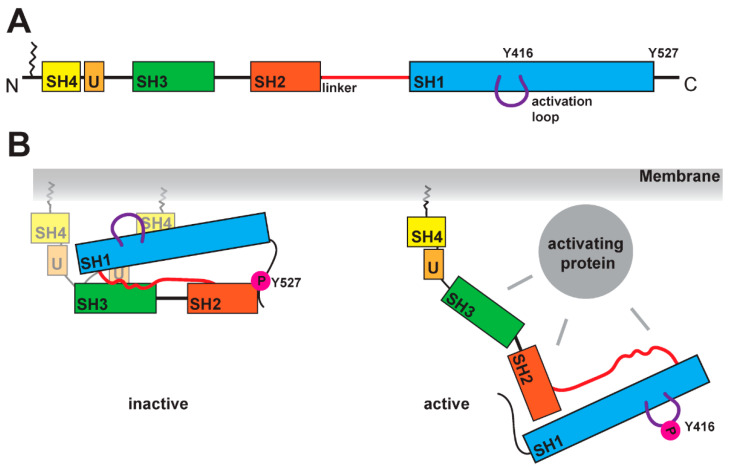
Figure 1.
Schematic of SFK domains and activation mechanism. (A) SFKs are organized in several domains: the SH4 domain (in yellow), a unique domain (in light orange), the SH3 domain (in green), the SH2 domain (in dark orange), the SH1 domain (in blue). At the N-terminal end, SFKs contain a lipid anchor, which is localizing the kinase at the membrane. The linker region between the SH2 and SH1 domain is crucial for the activation mechanism. A further structural feature is the activation loop within the SH1 domain, which contains a tyrosine residue (for Src Y416) that is phosphorylated in the active state of the kinase. (B) Comparison of inactive and active states of SFK. In the inactive conformation, the tyrosine in the activation loop is not phosphorylated, while the tyrosine (Y527 for Src) at the C-terminus carries a phosphate residue as it binds to the SH2 domain. The closed conformation is stabilized by the linker region binding to the SH1 and SH3 domains simultaneously. SH4 and unique domain seem to be more flexible, and recent studies found binding of the SH4 domain to the SH1 domain [32] (two possible conformations are shown). The open conformation is induced by the binding of an activating protein, which can interact with the SH3, SH2, SH1 domains and the linker. This active conformation shows phosphorylation of the tyrosine (for Src Y416) in the activation loop and dephosphorylation of the tyrosine in the C-terminus (for Src 527).