| Summary of the Compound N-Acetylcysteine | |||||
| Drug indication NAC is used mainly as a mucolytic and in the management of acetaminophen (paracetamol) overdose. | |||||
Chemical structure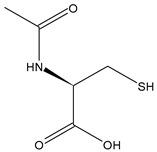
|
Molecular formula C5H9NO3S |
Synonyms | |||
| |||||
| pKa 3.24: –COOH and 9.5: –SH | |||||
| Molecular weight 163.2 g/mol |
Protein binding 66–97% (usually to albumin) |
WHO essential medicines Antidotes and other substances used in poisonings. |
|||
| Drug classes | Therapeutic uses | Taste Characteristic sour taste |
|||
|
|
||||
| Color/Form White crystalline powder | |||||
| Odor Slight acetic odor | |||||
| Drug warnings | |||||
| |||||
| Absorption | |||||
| |||||
| Metabolism | |||||
| |||||
| Half-life | Clearance | Volume of distrib | Excretion | ||
|
0.11 L/h/kg | ution 0.47 L/kg |
|
||
| Dosage forms | Overdosage | ||||
|
Single intravenous doses of NAC that were lethal:
|
||||
| Drug–drug interactions | |||||
| |||||
| Adapted from PubChem (2021) [31] and DrugBank (2021) [32]. | |||||

An official website of the United States government
Here's how you know
Official websites use .gov
A
.gov website belongs to an official
government organization in the United States.
Secure .gov websites use HTTPS
A lock (
) or https:// means you've safely
connected to the .gov website. Share sensitive
information only on official, secure websites.