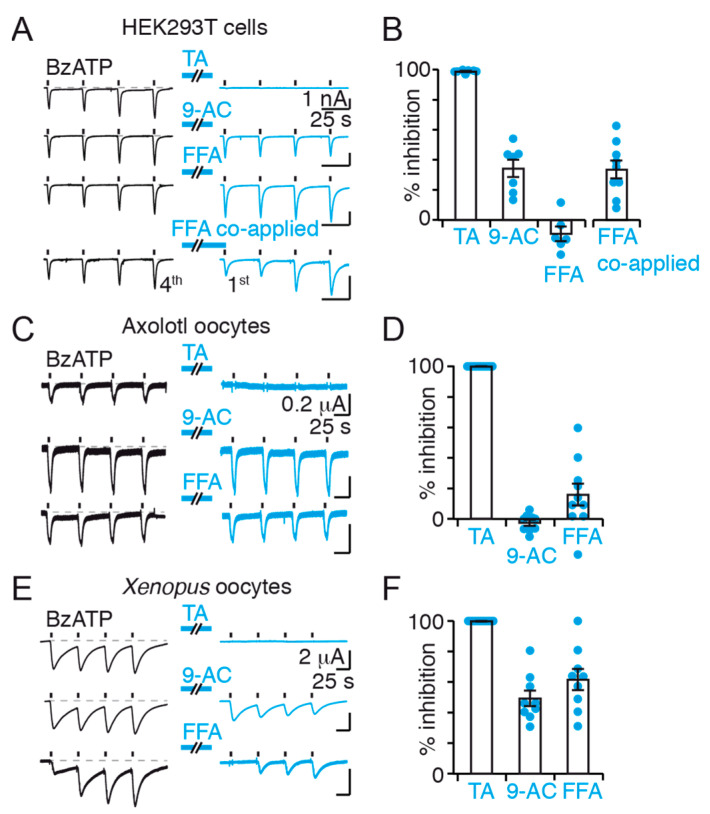
Figure 2.
Functional effects of CaCC inhibitors on P2X7 currents in HEK293T cells, Axolotl and Xenopus oocytes. (A) Whole-cell currents evoked by repeated 2 s applications of 10 μM BzATP (black bars) before and after 60 s perfusion of TMEM16 inhibitors (blue bars) in HEK293T cells transiently expressing rP2X7. In the case of FFA co-applied, perfusion was followed by a co-application with 10 μM BzATP. (B) Summary of whole-cell inhibition (n = 7 cells for TA, 7 for 9-AC, 6 for FFA, and 9 for FFA co-applied). Inhibition is calculated by comparing current amplitude of the fourth application before and first application after perfusion. Bars represent mean ± SEM. (C) Whole-cell BzATP-evoked currents recorded by TEVC of Axolotl oocytes expressing rP2X7 before and after application of TMEM16 inhibitors using the same protocol as in panel A. (D) Summary of inhibition in Axolotl oocytes calculated as in panel B (n = 10 oocytes for TA, 9 for 9-AC, and 10 for FFA). Bars represent mean ± SEM. (E) Whole-cell BzATP-evoked currents recorded by TEVC of Xenopus oocytes expressing rP2X7 using the same protocol as in panel A. (F) Summary of inhibition in Xenopus oocytes calculated as in panel B (n = 10 oocytes for TA, 9 for 9-AC, and 9 for FFA). Bars represent mean ± SEM. CaCC inhibitor concentrations were 20 μM TA, 1 mM 9-AC and 100 μM FFA. Data were recorded at −60 mV.