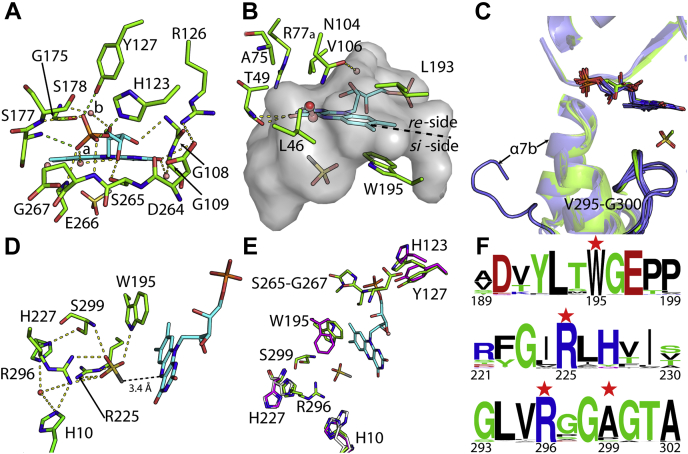
Figure 5.
Residues involved in flavin and MS−binding and a potential dioxygen-binding site.A, extensive polar contacts are made to the phosphate head and hydroxyls of the ribityl arm. B, Isoalloxazine interacting residues are shown. Residues of the dioxygen reactivity motif generate a partial pocket at the re-face of FMN where dioxygen is proposed to bind. Dioxygen is modeled in red sphere and stick representation from alignment of the FMN isoalloxazine ring of MsuD with the FMN of O2-bound RutA (Protein Data Bank ID 6SGG). The active site cavity is displayed as a gray surface. C, orientations of the ribityl arm and the loop of V295-G300 show alternate conformations within different chains of binary-soak MsuD (blue) in comparison with ternary-MsuD (green). An alternative orientation for α7b of the lid region in chain H is observed to direct the active site lid away from the FMN-binding site. D, MS− binding positions the methyl group of MS− approximately 3.4 Å from the N5 of FMN. E, an overlay of ternary-MsuD (chain A), binary-MsuD (FMN-bound chain A and unliganded chain H), and unliganded MsuD (chain A) shows residues that have alternate conformations when FMN is not present. Colors are as follows: ternary-MsuD carbons, green; binary-MsuD carbons, white; unliganded-MsuD carbons, magenta; FMN carbons, cyan; MS− carbon, gray; oxygen, red; nitrogen, blue; sulfur, yellow; phosphorus, orange; and waters, pink. F, sequence logos are shown for the sulfonate-binding motif of flavin-dependent alkanesulfonate monooxygenases with red stars for interacting residues.