Figure 6.
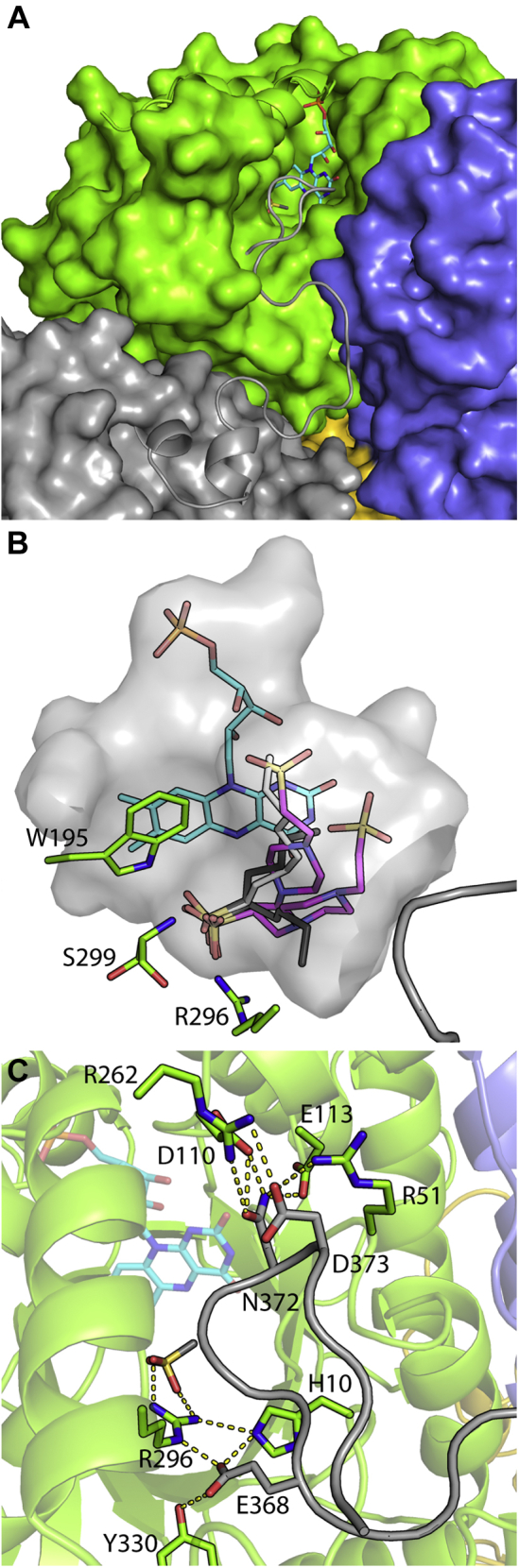
C terminus interactions within ternary-MsuD.A, the active site of MsuD chains A and B are displayed as green and blue surfaces with ribbons corresponding to the active site lid and the C terminus of chain D (gray). An identical interaction is observed for all chains in ternary-MsuD. B, example poses of docked C5 (gray) and C8 (light gray) sulfonates, and the buffer Pipes (magenta), position the sulfonate moiety similarly to MS−, but with variable conformations of the substrate. The active site cavity is displayed as a gray surface with a gap where the C terminus encloses the active site. R296 is omitted for clarity. Hepes and Mops docking results had similar placements of the sulfonate moiety but are omitted for clarity as both represent smaller buffer molecules compared to Pipes. C, polar interactions of the C terminus are shown.
