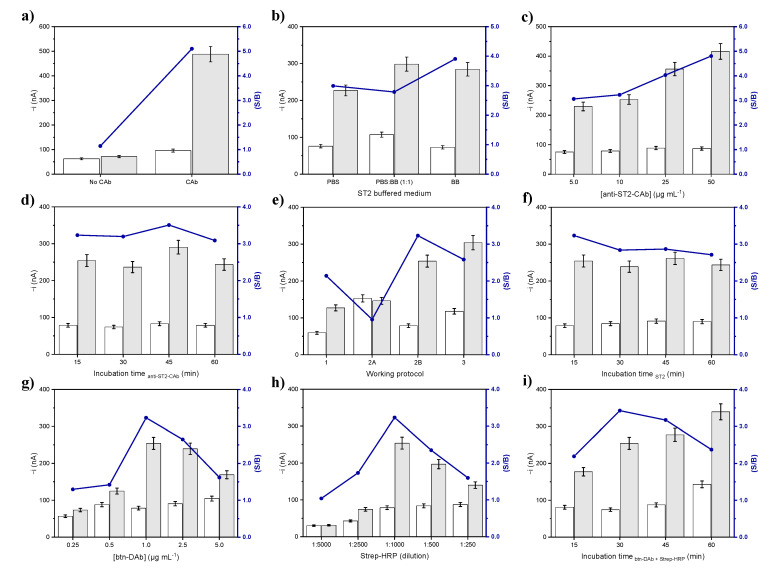
Figure 2.
Feasibility of the immunosensor design (a), and effect of the working variables on the amperometric response provided by the developed immune platform (b–i): (b) different buffered medium for the preparation of ST2 standard solutions; (c) concentration and (d) incubation time of anti-ST2-CAb; (e) steps involved in the working protocol; (f) incubation time with ST2; (g) concentration of btn-DAb; (h) Strep-HRP dilution; and (i) incubation time of the mixture containing btn-DAb and Strep-HRP. Amperometric responses measured in the presence of 0.0 (white bars) and 1000 (a) or 500 (b–i) (grey bars) pg mL–1 ST2 and the resulting signal-to-blank ratios ((S/B), blue dots and lines).