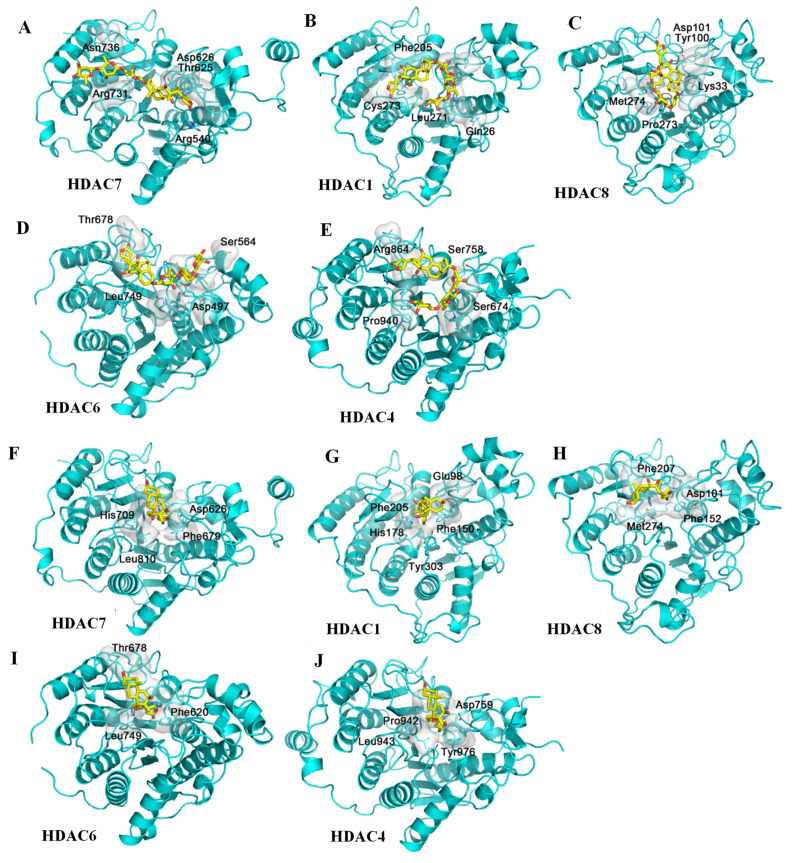
Figure 5.
Docking profiles for digoxin (yellow) and histone deacetylases HDAC7 (A), HDAC1 (B), HDAC8 (C), HDAC6 (D), and HDAC4 (E); and digoxigenin (yellow) and HDAC7 (F), HDAC1 (G), HDAC8 (H), HDAC6 (I), and HDAC4 (J). The HDAC family has three classes and over 10 members in humans, but the structures of these proteins are notably similar, with the same binding site being at the center of each HDAC domain. Five crystal structures of different HDACs were obtained as the receptors for the docking of digoxin and digoxigenin. The center pocket of HDACs is too small for the steroid motif and the lactone unit of digoxin to be inserted, and thus, digoxin could not bind to HDACs (A–E), even though two different sizes of docking boxes were used to carry out docking. However, the docking profiles for digoxigenin and HDACs are greatly improved (F–J) when compared with those for digoxin.