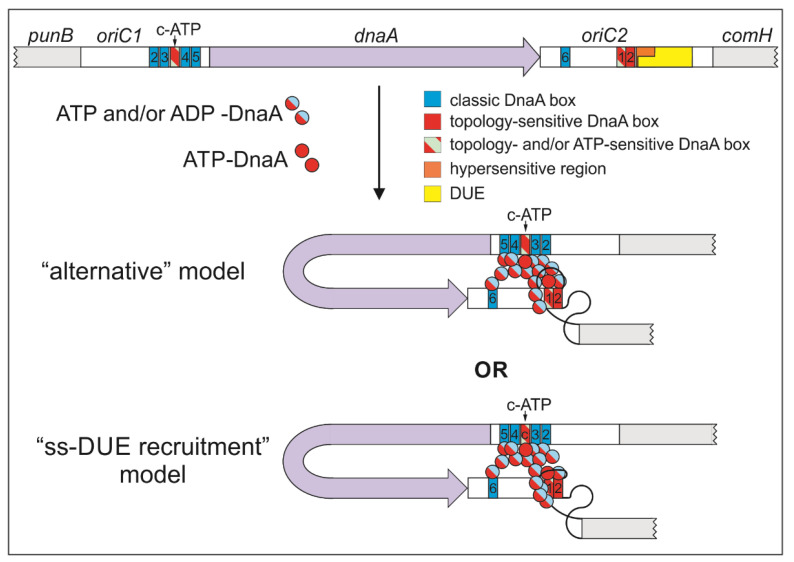
Figure 8.
Schematic representation of possible H. pylori orisome structures on bipartite oriC. Boxes c-ATP and ts1 are bound exclusively by ATP–DnaA, whereas both DnaA forms, ADP-bound and ATP-bound, interact with classic DnaA boxes. The nucleotide status of the DnaA (ATP or ADP) forming the complete oligomer is unknown. The binding of DnaA to dsDNA leads to DNA unwinding. DnaA bound to ssDNA stabilizes the interaction of the protein with DnaA box ts1 either by recruitment of additional DnaA molecules (“alternative” model) or by simultaneous ssDNA and dsDNA binding (“ss-DUE recruitment” model). Whether or not there is additional stabilization/orientation of ssDNA–DnaA oligomer by DnaA bound to oriC1 of H. pylori is also an open question.