FIGURE 5.
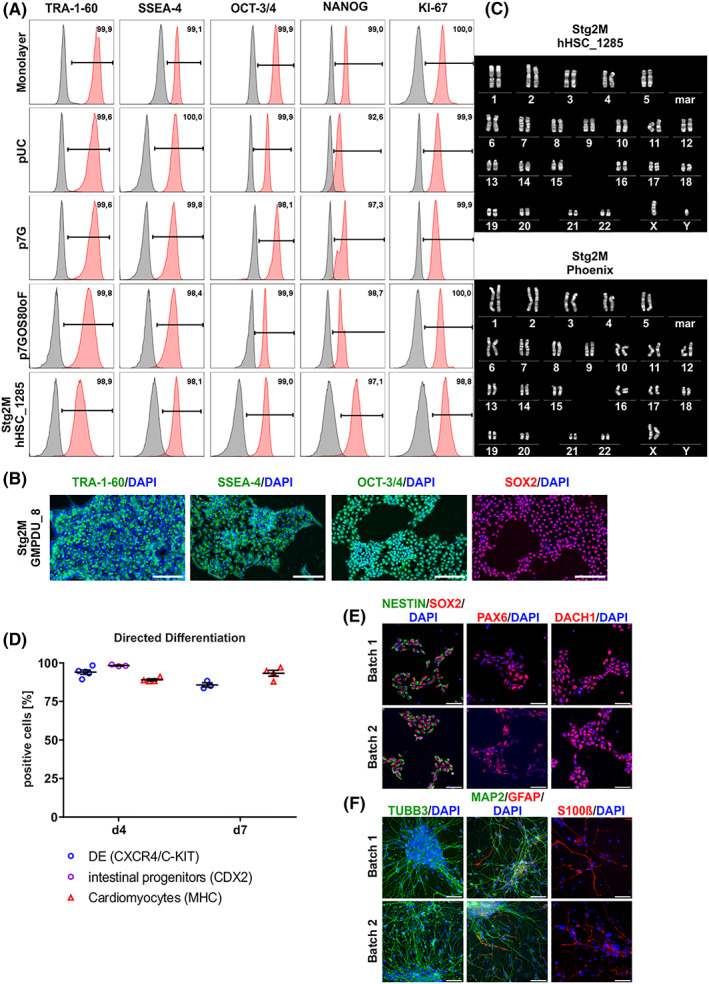
A, Pluripotency marker expression of cells harvested at process endpoints. Exemplified flow cytometry analysis plots showed that the majority of cells harvested at process endpoint (day 7) expressed pluripotency associated surface markers SSEA‐4 and TRA‐1‐60 as well as transcription factors OCT‐3/4 and NANOG at levels equivalent to monolayer pre‐cultures used for bioreactor inoculation (isotype controls shown in gray). Additionally, no decrease in proliferation marker KI‐67 could be measured. All Data displayed in (A) was generated with the cell line hHSC_1285_iPS2. B, Process‐derived hPS cell aggregates were dissociated and plated down. Representative pictures of immunofluorescence staining of day 7 derived cells stained for pluripotency markers TRA‐1‐60, SSEA‐4, OCT‐3/4, and SOX2. Positive staining is shown in green/red. Isotype controls confirmed staining specificity (not shown). DAPI stained nuclei in blue. Scale bars = 200 μm. All data displayed in (b) was generated with the cell line GMPDU_8. C, Karyotype of cells cultured for 7 days under the Stg2M conditions displayed for the cell lines hHSC_1285_iPS2 and Phoenix. D, Process‐derived hPS cell aggregates of GMPDU_8 cultured under Stg2M conditions for 4 and 7 days were directed differentiated into definitive endoderm (DE; based on double positive CXCR4/C‐KIT) and intestine (based on CDX2, only on day 4) as well as cardiomyocytes (based on MHC). Characterization of small molecule neural precursor cells (smNPCs) derived from high density hPSC bioreactor cultures. E, Samples from two independent high density hPSC bioreactor batches were differentiated into smNPCs and subsequently immunostained for markers of neural stem cells/progenitors including SOX2, NESTIN, and PAX6 as well as the neural rosette‐associated transcription factor DACH1. F, Upon growth factor withdrawal for 4 weeks differentiated smNPC cultures were stained for the neuronal markers TUBB3 and MAP2 as well as the glial markers GFAP and S100beta. Nuclei were counterstained with DAPI. Scale bars: 50 μm. All data displayed in (D)‐(F) was generated with the cell line GMPDU_8
