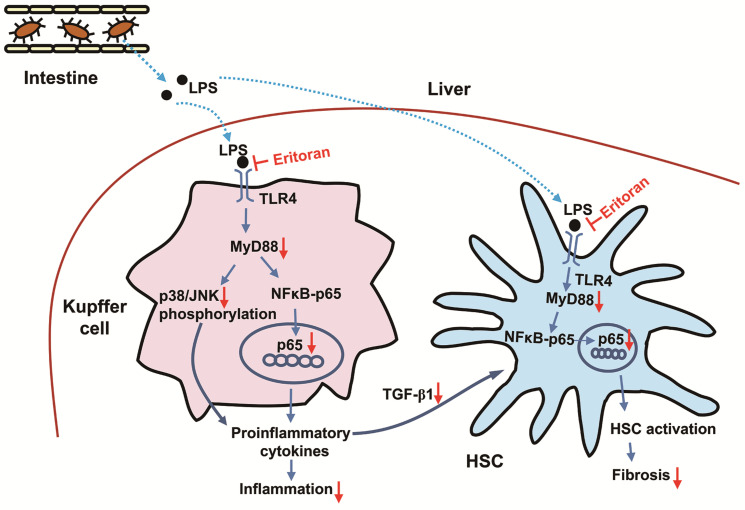
Figure 12.
The proposed scheme of the regulation mechanism of eritoran in mice with chronic liver injury. The increased lipopolysaccharide (LPS) due to the increased gut permeability in the fast-food diet-fed or tetrachloride-injured mice [45,46] entered the liver and bound toll-like receptor 4 (TLR4) on the Kupffer cells or hepatic stellate cells (HSCs). In Kupffer cells, LPS activated myeloid differentiation factor 88 (MyD88), leading to the NF-κB p65 nuclear translocation and increased phosphorylation of p38 and c-Jun N-terminal kinase (JNK), which contributed to increased production of proinflammatory cytokines and inflammation. The secreted transforming growth factor-β1 (TGF-β1) from Kupffer cells could promote HSC activation. On the other hand, LPS binding to TLR4 of HSCs could also activate MyD88, leading to the NF-κB p65 nuclear translocation, which contributed to HSC activation and liver fibrosis [10,11,12,39]. Eritoran blocked the TLR4 signaling pathway by competing with LPS for binding sites on Kupffer cells and HSCs, leading to downregulation of the MyD88-dependent NF-κB and JNK/p38 pathways, which contributed to attenuation in hepatic inflammation and fibrosis. Blue arrow: upregulation; red arrow: downregulation.