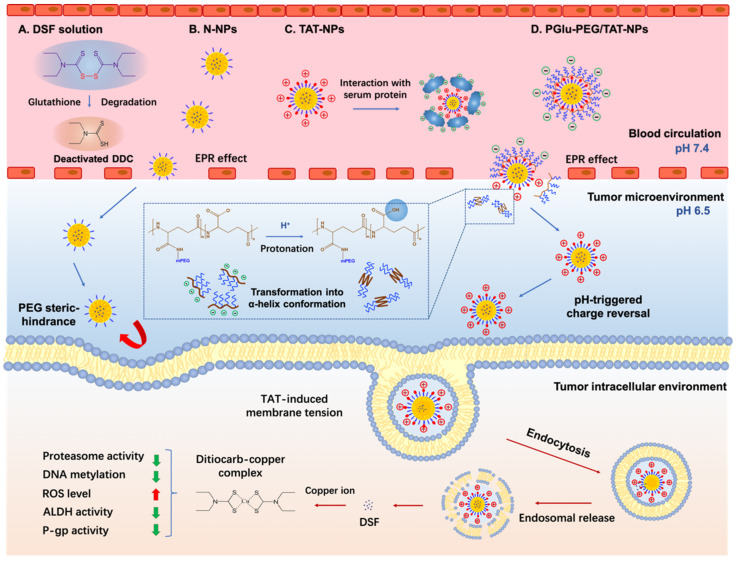
Scheme 1.
Schematic illustration of tumor microenvironment-responsive DSF-loaded nanoparticles for enhanced stability and antitumor efficiency based on a pH-triggered charge-reversal mechanism. DSF in solution or TAT-NPs could interact with glutathione or serum protein, leading to short half-life in blood circulation. The PEG steric hindrance on N-NPs could limit the intracellular uptake of DSF. PGlu-PEG/TAT-NPs could target to tumor sites and dePEGylate to TAT-NPs due to the protonation of PGlu-PEG to accomplish pH-triggered charge reversal in the tumor microenvironment; TAT-induced cellular uptake increases the accumulation of intracellular DSF, which could form ditiocarb–copper complex to inhibit tumor progression through multiple pathways. N-NPs: DSF-loaded naked nanoparticles, composed of DSF, medium-chain triglycerides, macrogol 15 hydroxystearate and lecithin; TAT-NPs: DSF-loaded cationic nanoparticles, composed of HS-PEG-TAT and N-NPs; PGlu-PEG/TAT-NPs: DSF-loaded shell/core composite nanoparticles, composed of PGlu-PEG and TAT-NPs. DDC, diethyldithiocarbamic acid; ROS, reactive oxygen species; ALDH, aldehyde dehydrogenase; P-gp, P-glycoprotein.