Figure 9.
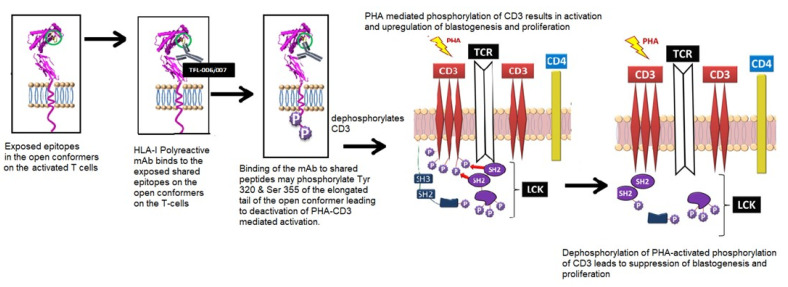
A model illustrating the possible mechanism underlying phytohemagglutinin (PHA) activation of T-cells and the suppression of activated T-cells mediated by HLA-I polyreactive mAbs (TFL-006 and TLF-007) and possibly by IVIg. The model is based on a model proposed by Mustelin, Vang, and Bottini [77] for T-cell activation. The CD3/T-cell receptor (TCR)/CD4 structure on the lipid raft (pink zone) of the bi-layered lipid membrane on the non-phosphorylated, non-activated CD4+ T-cells is illustrated. The lymphocyte-specific protein, tyrosine kinase (LCK), induced phosphorylation of tyrosine-based activation in the cytoplasmic domain of CD3, which led to the activation of transcription factors and the transcription of cell surface molecules such as interleukin (IL)-2Rα and open conformers of HLA class I. SH-1, SH-2, and SH-3 represent family members of Src homology; they are involved in mediating the cytoplasmic domain of CD3. Further activation of the tyrosyl-phosphorylated motifs and then interaction with SH-1 domains within the protein kinase LCK led to further signaling function [77]. Importantly, the exposure of shared amino acid sequences of all the HLA open conformers is indicated by a blue circle. It is this site that is recognized by TFL-006 and TFL-007. Possible interactions and consequences of recognition of the shared peptide sequences by the HLA-I polyreactive IgG mAbs are illustrated in three steps: first, the exposure of the shared peptide sequence on the open conformer; secondly, recognition of the shared epitopes on the open conformer by the mAbs; thirdly, possible phosphorylation of the elongated cytoplasmic tail of open conformers. That elongation resulted in the exposure of cryptic tyrosine (Tyr320) and serine (Ser355) residues in the cytoplasmic tail. It might have been the binding of the mAbs to the shared peptide sequences that initiated the phosphorylation, leading to signal transduction. A final step involved initiation of dephosphorylation of the cytoplasmic domain of CD3, resulting in arrest of activation or suppression. That seemed plausible, as the phosphorylation was known to be reversible.