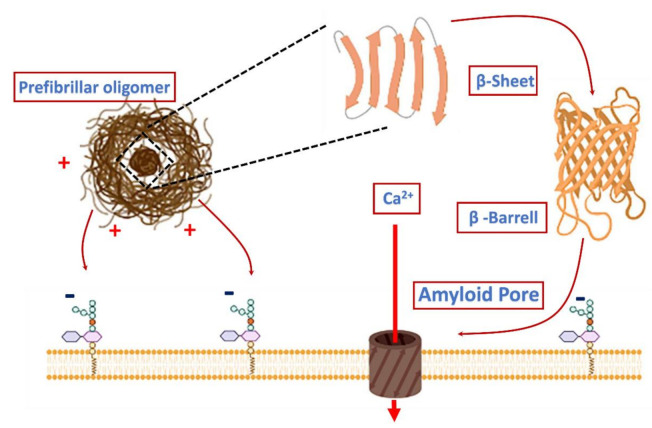
Figure 4.
The possible pore formation mechanism by an amyloid PFO. According to the most recent data collected in the literature, the typical amyloid PFO may have the shape of a sphere with a stiff hydrophobic core, partially structured in a β-sheet. The surface of the sphere exposes both polar and hydrophobic protein sequences. The first, exposed to the solvent and likely charged, are responsible for interaction with charged parts of the membranes, such as the GM1 forming the “lipid-rafts”. After the contact, the partially ordered PFO hydrophobic core, in order to minimize the hydrophobic mismatch with the membrane backbone, forms a permeable amyloid pore assuming a β-barrel conformation favored by the ordered structure of the “lipid-rafts”.