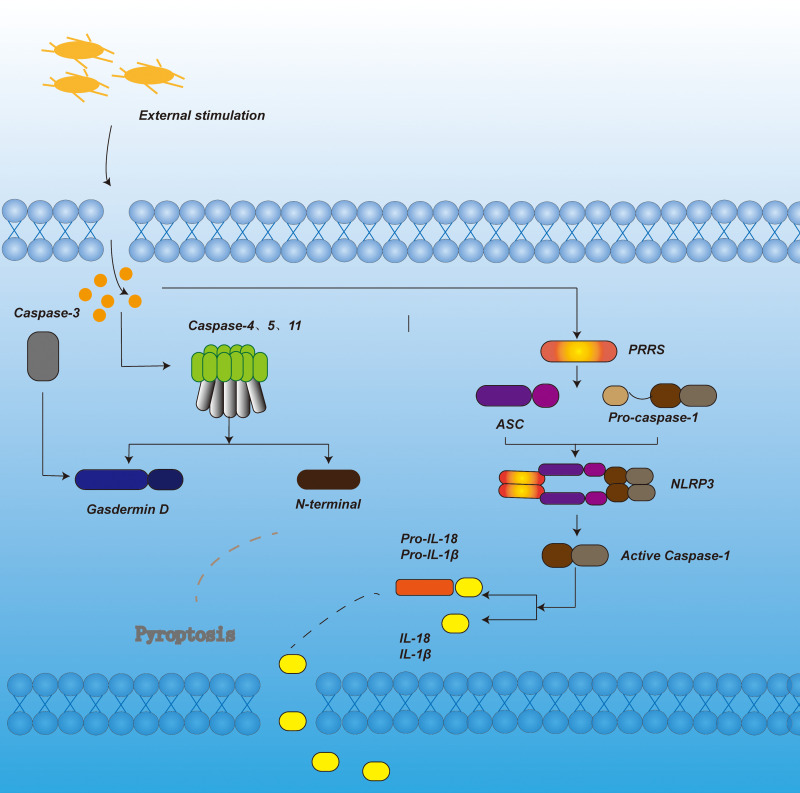
Figure 1.
In the canonical pyroptosis signaling pathway, under the stimulation of bacteria, viruses and other signals, the pattern recognition receptor in the cell acts as a sensor to recognize these signals. Through the adaptor protein ASC, it binds to the precursor of Caspase-1 to form a multi-protein complex and activate Caspase-1. Activated Caspase-1 cleaves Gasdermin D to form peptides containing the nitrogen-terminal active domain of Gasdermin D, induce cell membrane perforation, cell rupture, release of contents, and cause inflammation. On the other hand, activated Caspase-1 cleaves the precursors of IL-1β and IL-18 to form active IL-1β and IL-18, which are released to the outside of the cell to recruit inflammatory cells to aggregate and expand the inflammatory response. In the non-canonical pyroptosis signaling pathway, under the stimulation of bacteria and other signals, Caspase-4, 5, and 11 are activated. The activated Caspase-4, 5, and 11 cleave Gasdermin D to form a peptide containing the nitrogen-terminal active domain of Gasdermin D. On the one hand, it induces cell membrane perforation and cell Rupture, release the contents and cause inflammation. On the other hand, it induces the activation of Caspase-1, cleaves the precursors of IL-1β and IL-18, forms active IL-1β and IL-18, and releases them to the outside of the cell, recruits inflammatory cells to aggregate, and expands inflammation reaction. In apoptotic-like pyrolysis, caspase-3 cuts GSDME, promotes the recruitment of GSDME-N domain to the cell membrane, induces the formation of cell membrane pores, and leads to pyroptosis.