Abstract
Methods
Seventeen patients aged 18 to 55 years with symptomatic full-thickness cartilage lesions on either patella or trochlea were treated with matrix autologous chondrocyte implantation (MACI) or microfracture (MF). Both procedures combined with unloading/realigning techniques. Clinical assessment and T2-mapping were evaluated at 48-months.
Results
Clinically results from pre-op to 48-months improved significantly in MACI and MF for Lysholm (p = 0.001, p = 0.001), IKDC-S (p = 0.001, p = 0.002), KOOS-P (p = 0.000, p = 0.002), KOOS-DLA (p = 0.002, p = 0.003), KOOS-Sports/Rec (p = 0.000, p = 0.004), KOOS-QoL (p = 0.000, p = 0.003), KOOS-symptoms (p = 0.001, p = 0.020), and Kujala (p = 0.000, p = 0.01), respectively. Tegner was significant between baseline and 48 months only for MACI (p < 0.008) compared with MF (p = 0.25). No significant difference was observed between groups for any score at 3, 12, 24, and 48-months (p > 0.05). T2-mapping values improved significantly over time in MACI compared with MF at 24 months (39.35 vs. 50.44, p = 0.007) and 48 months (36.54 vs. 48.37, p = 0.005). When comparing control values to MACI at 12-m (p = 0.714), 24-m (p = 0.175), and 48-m (p = 0.097), no significant difference was found. MOCART (Magnetic Resonance Observation of Cartilage Repair Tissue) score comparison gave no statistical difference between groups.
Conclusions
Clinically both techniques improved significantly over time. However, quantitative assessment showed that only newly formed tissue with MACI technique improves significantly since 12-months and maintains stable values compared with native cartilage until 48-month follow-up. MF results were never comparable to those native values. Level of evidence II.
Keywords: cartilage repair, patellar chondral lesions, trochlear chondral lesions, chondrocyte implantation, microfracture
Introduction
Cartilage lesions are commonly found during routine knee arthroscopy. Different studies have reported the presence of high-grade focal chondral defects in 11% to 20% of knee arthroscopies, and among these injuries 11% to 23% were located in the patella and 6% to 15% in the trochlea.1-3 Cartilage lesions in the patellofemoral joint (PFJ) represent a challenge to treat not only because of the problem of poor vascularity of cartilage and its limited capacity for self-repair but also because its complexity and its high axial and shearing forces.2,4-7 Both acute traumatic events and chronic PFJ misalignment can cause this entity. Different factors can produce maltracking, which results in overload of the PFJ and must be addressed as part of cartilage repair treatment.8-11 Although there are different techniques for cartilage repair there is no consensus on which technique provides better results in this complex joint.5-8
Microfracture (MF) has the best results in young patients (<40 years old) with small lesions of the femoral condyles. Penetration to the subchondral bone leads to bleeding and subsequent clot formation with synthesis of hyaline-like cartilage initially; however, over the time it becomes more fibrous and deteriorates as a result of suboptimal biomechanical properties of the repaired tissue.5,12-16 Matrix autologous chondrocyte implantation (MACI) is performed in a 2-step process in which a patient undergoes knee arthroscopic surgery to obtain a biopsy of healthy cartilage. Chondrocytes from this biopsy sample are then cultured over several weeks and implanted into a collagen or hyaluronan-based scaffold before being cut to the shape and size of the patient’s chondral defect and fixed to the defect with fibrin glue or other methods. 17 Although costly and a more demanding procedure, it has a theoretical benefit of being able to give a more chondral-like tissue with less fibrocartilaginous proportion compared with MF.
The primary objective of this study is to do a quantitative assessment through T2-mapping and MOCART (Magnetic Resonance Observation of Cartilage Repair Tissue) score (and as secondary objectives a clinical and a second look evaluation) of patients with cartilage lesions in the patella and trochlea, comparing the 2 arthroscopic techniques (MF vs. MACI) over a 4-year period in a controlled comparative cohort study; according to our hypothesis, MACI provides a better quality of the repair tissue than MF when image assessment is evaluated by T2-mapping and MOCART in the PFJ, maintaining comparable signal intensity than native cartilage at 4-year follow-up.
Methods
A controlled comparative cohort study was approved by the institutional committee. Patients treated with MACI or MF during the period of 2010 to 2013 were included, with ages between 18 and 55 years and symptomatic full-thickness cartilage lesions on either patella or trochlea, with or without misalignment. Concomitant anterior cruciate ligament (ACL) or meniscal lesions were treated at the first surgery in both groups. Presence of any type of arthritis, previous treatment of the cartilage lesions, and failure to follow the rehabilitation protocol were exclusion criteria.
Clinical evaluation was performed preoperatively and postoperative at 3-, 12-, 24-, and 48-month follow-up. The quality of the repaired tissue was assessed by T2-mapping magnetic resonance imaging (MRI) at 3, 12, 24, and 48 months postoperative. Second look arthroscopy was performed at 12 months for ICRS (International Cartilage Repair Society) classification in both groups.
Surgical Technique
Patients treated in both groups underwent the index procedure (ACL or meniscal lesion), and at the same surgery cartilage damage was treated with MACI or MF. Any associated PFJ misalignment or hyperpresion was addressed before MF performance, with a Fulkerson’s osteotomy in patients with TT-TG >15 mm, and a lateral retinacular release with medial plication in those <15 mm.
Microfracture
In patients treated with MF the articular cartilage lesion was identified, measured, and debrided to leave stable and healthy walls of native cartilage. Straight and angled curettes were used to remove unstable cartilage edges and the calcified layer. A surgical 90° angled awl was used to create 4 mm depth holes, with 4 mm of distance between them, in the exposed bone either in the trochlea or patella.
Matrix Encapsulated Chondrocytes Implantation
Cartilage biopsy
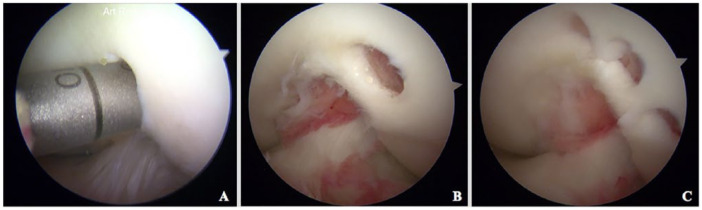
Depending on the size of the cartilage lesion, 2 to 3 osteochondral biopsies were taken from a non–weight-bearing area adjacent to the lateral intercondylar notch. An osteochondral graft harvester of 4 mm diameter was used (COR; DePuy Mitek, Raynham, MA) ( Fig. 1 ). The biopsies were placed in sterile tubes containing Dulbecco’s modified Eagle medium-F12 medium with 1% antibiotics/antimycotic agents. Samples were sent to the biotechnology laboratory for chondrocyte isolation, in-vitro expansion, and cell-polymer scaffold formation as described by Masri et al. 18
Figure 1.
Osteochondral biopsy harvesting. (A) An osteochondral harvester (COR; DePuy Mitek, Raynham, MA) was used to get one to three 4-mm diameter biopsies in a non–weight-bearing area adjacent to the intercondylar notch (B and C).
Chondrocyte isolation and expansion
Under sterile conditions in a laminar flow hood, cartilage was separated from bone by sharp dissection and fragments were digested with type 2 collagenase during 4 to 5 hours. Chondrocytes were counted, and viability was assessed by trypan-blue stain. Samples of cells in suspension were sent to a laboratory for microbiological evaluation (bacteria, fungi, Mycoplasma) for quality control. Then cells were seeded into a T25 culture flask at a density of 2.5 × 104 cells with culture medium (Dulbecco’s modified Eagle medium-F12; GIBCO, Grand Island, NY) supplemented with 1% antibiotic-antimycotic and 10% autologous serum. Chondrocytes were expanded until 90% confluence and were then digested with trypsin and re-seeded for cell expansion until passage 3. At the beginning of the third passage, 50% of the cells were seeded in petri dishes with conventional media supplemented with ascorbic acid to induce monolayer formation while the remaining cells were expanded in T25 flask without ascorbic acid to obtain a pellet of chondrocytes.
Construct formation
Once chondrocytes in passage 3 reached 90% to 100% confluence, cells in the T25 culture flask were digested to obtain a pellet of chondrocytes. Chondrocytes expanded in monolayer were released from petri dishes. A piece of 8 mm diameter polyglycolic acid scaffold (Neoveil Sheet, Gunze Medical Division, Japan) was placed over this monolayer and the pellet of chondrocytes was added to this scaffold. Finally, polyglycolic acid scaffold plus chondrocyte pellet were enveloped with the borders of monolayer as a crepe. This construct was cultured for 1 week to allow cell adherence and matrix production.
Arthroscopic Implantation
In a second arthroscopic procedure, the constructs were implanted for the MACI group. With the patient supine on the operating table and under regional anesthesia, the knee was prepared and draped in a conventional manner. A tourniquet was placed around the proximal thigh, although normally it was not insufflated. A conventional longitudinal anterolateral portal was established for arthroscopic examination of the joint using a superolateral portal for irrigation. The articular cartilage injury was identified, measured, and prepared for construct implantation.
Chondrocyte implantation in the trochlea
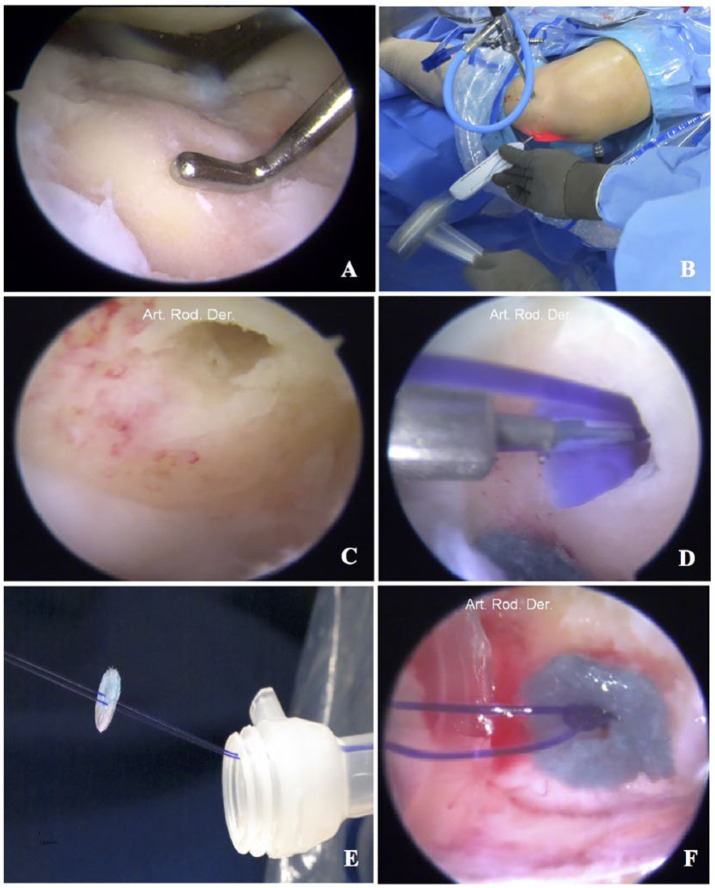
Cartilage lesion was measured and debrided to leave stable walls ( Fig. 2A ). When the lesion was in the medial trochlea, an oblique anteromedial portal was established over the lesion to have perpendicular access. If the lesion was on the lateral trochlea, the anterolateral portal was extended proximally or distally to allow perpendicular access. A 2-mm hole was made in the center of every centimeter of cartilage lesion and an absorbable 1.9-mm anchor (MINILOK, Depuy Synthes Mitek, Raynham, MA) with 0-PDS suture (Ethicon, Somerville, NJ) was inserted through the anteromedial or anterolateral portal ( Fig. 2B , C , and D ). The cell-scaffold disk was prepared on the side table. An 8-mm transparent cannula was then inserted through the portal directly over the lesion, and the sutures from the anchor were pulled outside the joint through an arthroscopic cannula ( Fig. 2E ). The anchor sutures were passed in the construct through 2 needles (20 G × 32 mm), and the construct was slid into the joint to place it in the bottom of the cartilage lesion. A self-locking arthroscopic sliding knot was used to fix the implant ( Fig. 2F ). Once the construct was sitting in place at the bottom of the lesion, the knot was tightened by pulling on the wrapping limb of the suture, and 2 additional half-hitch knots were tied with the assistance of a knot pusher. The sutures were then cut flush to the knot and the cannula was retrieved. Stability of the implant was then tested with the probe, and the knee was taken through a range of motion to verify the stability and permanence of the implant at the repair site.
Figure 2.
Matrix chondrocyte implantation in trochlear lesions. (A) Cartilage lesion is measured and debrided with a curette to leave stable walls. (B, C, and D) A 1.7-mmi hole was made in the center of the lesion and an absorbable anchor charged with 0-PDS suture is inserted. (E) The implant slided into the joint through a cannula and the is fixed with self-locking arthroscopic sliding knot; 2 or 3 additional half-hitch knots (F).
Chondrocyte implantation in the patella
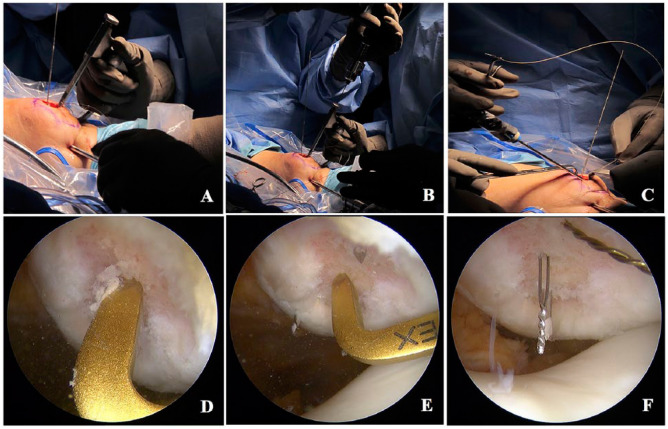
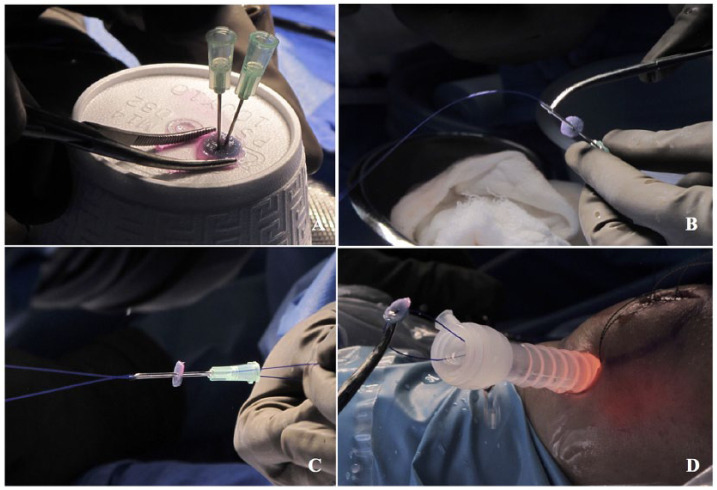
Treatment of cartilage lesions in the patella with ACI technique were performed with the use of the ACL tibial guide at different grade of angulation. Standard arthroscopy evaluation was done to evaluate additional lesions. The chondral lesion was identified, measured, and debrided and the ACL tibial guide (ACUFEX; Smithnephew, Andover, MA) was introduced either through the medial or lateral portal to have easy access to the lesion ( Fig. 3A ). Using the elbow aimer of the ACL tibial guide, the angle of the aimer was adjusted depending on the better position of the tip over the center of the lesion ( Fig. 3D ). Two holes were drilled with a cable wire (Kirschner 0.062 inch) from the anterior cortical of the patella to the subchondral bone ( Fig. 3B and E ); the holes were placed in the center of every 10 mm of the cartilage lesion. The cable wires were left in place while the ACL tibial guide was removed from the joint, a 15-mm skin incision was performed anterior to the patella connecting the 2 cable wires ( Fig. 3C ), and deep dissection was performed until the periosteum. Once the wires were identified, they were removed with the drill, and a chia (CHIA PERCPASSER, Suture Passer Depuy Synthes Mitek, Raynham, MA) was inserted in every hole from outside to inside until the chips are visible and accessible in the joint space ( Fig. 3F ); we advanced the chia tip down to grab it with a grasper from either medial or lateral portals. In the back table the construct was prepared, and 2 percutaneous needles (20 G × 32 mm) were inserted in the center of the construct from the base (polyglycolic acid) to the surface area (matrix encapsulated chondrocytes) ( Fig. 4A ). One 0-PDS suture was folded, and its ends were passed in the construct through the needle tips ( Fig. 4B and C ). Once PDS was placed in the center of the construct the needles were removed. A 10-mm cannula was placed in the chosen portal where the chias were grabbed, and every end of the 0-PDS suture with the construct was introduced in the loop of the chias and then those are pulled to introduce the construct into the joint ( Fig. 4D ). Once the construct was placed in the bottom of the lesion ( Fig. 5A ), a non-sliding knot was made and tied over the anterior cortical of the patella outside the joint ( Fig. 5B ). Steps were repeated if more than one construct was needed. Portals and accessory incision were closed in the traditional manner.
Figure 3.
Arthroscopic chondrocyte implantation in patella. (A and D) The ACL tibial guide is introduced by the portal that permits better position to the center of the lesion. (B and E) Two holes are drilled from the anterior cortical of the patella to the subchondral bone at the center of the lesion. (C and F) An anterior skin incision is made over the patella; deep direction is necessary to visualize the entrance of both cable wires. Cable wires are removed with the drill and a chia passer is inserted in every hole until it is visible into the joint space.
Figure 4.
Preparation of the chondrocytes construct with a 0-PDS suture. (A) Two needles (20 G × 32 mm) are inserted in the center of the construct leaving 2 mm of distance. (B and C) The ends of 0-PDS are passed the through the needles. (D) Once the PDS is placed in position, needles are removed, and the ends of the PDS are introduced in the loop of every chia passer and the construct is pulled slowly through a 10-mm cannula.
Figure 5.
Fixation of the construct. (A and B) Once the construct is placed in the bottom of the lesion, 3 to 4 sliding knots are tied over the anterior cortical of the patella. Notice that different to trochlear implantation in patellar technique the knots are out of the articular space.
Rehabilitation Protocol
After implantation, patients were included in a very strict rehabilitation protocol that started the same day of the procedure with cryotherapy, continuous passive motion from the first day after surgery up to 8 weeks (6 to 8 hours/day), no weight-bearing for 8 weeks, and progressive open-chain strengthening after a first isokinetic evaluation at 3 months after surgery. Continuous passive motion was started from 0° to 30° of flexion the same day of surgery during 2 weeks, and after this time 30° per week was added until 120° of flexion. This method was selected over straight-leg bearing due to certain patients having a concomitant ACL/meniscal lesion or misalignment treatment.
Second Look
Second-look arthroscopy was performed in all patients at 12 months. Three experienced arthroscopic surgeons performed a blinded evaluation using the ICRS classification system by independently watching the surgical video.
Statistical Analysis
Dimensional data were expressed as means and standard deviations. Qualitative data were presented in absolute numbers or percentages, or both. The Wilcoxon signed-rank test for paired samples was used to compare before and after preoperative clinical and MRI values to 48-month follow-up and comparisons between groups (P < 0.05 was considered significant). Student’s t test was performed for mean difference between continuous data. Software used for analysis: STATA 12® (StataCorp 2011).
MRI Assessment
MRI evaluation was performed preoperatively and before cell-construct implantation using T2-mapping and MOCART scores, and at 3, 12, 24, and 48 months postoperatively for T2-mapping and 48 months postoperatively for MOCART score. MRI was performed on a 1.5-Tesla clinical imaging system (GE Healthcare, Milwaukee WI), using an 8-channel HD knee array (GE Healthcare). Standard morphologic MRI evaluation was performed using a fast spin echo sequence in the axial, sagittal, and coronal planes. Images were acquired with repetition time of 1800 to 1450 ms, echo time of 30 to 40 ms, echo train length of 6, and spatial resolution of 256 mm (frequency), 256 mm (phase), 3 mm at 2 excitations.
The qualitative evaluation of cartilage repair was performed by 2 independent radiologists using the MOCART scoring system. The score consists of 9 variables: (1) degree of defect repair, (2) integration of border zone, (3) surface of the repair tissue, (4) structure of the repair tissue, (5) signal intensity of the repair tissue, (6) subchondral bone, (7) subchondral lamina, (8) adhesions, and (9) effusion.
T2-mapping (FuncTool 4.5.1, GE Healthcare, Little Chalfont, Buckinghamshire, UK) was performed to assess the biochemical integrity of native and repaired cartilage. The color map is coded to capture T2 values ranging from 25 to 95 ms. Quantitative T2-mapping was performed using a multislice multiecho pulse sequence. Eight echoes were sampled: sequential multiples of the first echo time (10 to 11 ms) at a repetition time of 800 ms and in-plane resolution of 384 mm (frequency), 256 mm (phase), 3 mm at 2 excitations. Data sets were analyzed (FuncTool 4.5.1; GE Healthcare). T2 values were calculated taking a region of interest (ROI) (2-6 mm) within a fixed area in the center of the repair (named ROI6) and normal cartilage (named ROI3).
Results
A total of 17 patients with aged between 18 and 55 years with symptomatic, posttraumatic, single or multiple (maximum 3) isolated defects (1-4 cm2) in the PFJ were included in the study.
Demographics
In MACI, 10 patients were enrolled with a mean age of 36 years (±3.92); 6 patients were male and 4 were female. The mean size of the lesion was 1.18 cm2 (±0.25). Six of 10 patients had additional diagnosis. The mean body mass index (BMI) was 26.12 (±2.37).
The 7 patients that received MF had a mean age of 39 years (±8.72); 4 were women and 3 were men. The mean size of the lesion was 1.21 cm2 (±0.27). Six patients had additional lesions. The mean BMI was 26.12 (±2.37) ( Tables 1 and 2 ).
Table 1.
Lesion Site and Additional Joint Lesions.
| Group | Patients | Trochlea | Medial Patella | Lateral Patella | Combined Cartilage Lesions | Additional Joint Lesions |
|---|---|---|---|---|---|---|
| MACI | 10 | 4 | 1 | 2 | 3 | 3 Lateral hyperpresion; 1 Patella dislocation; 2 ACL ruptures; 2 Meniscal lesions |
| MF | 7 | 4 | 1 | 2 | 0 | 2 Lateral hyperpresion; 1 ACL rupture; 1 Patella dislocation; 2 Meniscal lesions; 1 Other minor cartilage lesions |
MACI = matrix autologous chondrocyte implantation; MF = microfracture; ACL = anterior cruciate ligament; PFJ = patellofemoral joint. In the MACI group only 4 patients had pure cartilage lesions in the PFJ and the remaining 6 had combined joint lesions treated with different additional techniques. In the MF group all patients had other knee joint lesions that also received appropriate treatment combined with the corresponding cartilage repair technique.
Table 2.
Patient Lesions and Treatments Characteristics.
| Group/Patient | Lesion Site | Lesion Size (cm2) | Side | Concomitant Treatments |
|---|---|---|---|---|
| MACI | ||||
| 1 | Lateral and medial patella | 1.4 | Left | Lateral retinacular release and medial plication |
| 2 | Trochlea | 1.4 | Right | ACL reconstruction with hamstrings, medial meniscal repair |
| 3 | Medial patella | 1.3 | Left | |
| 4 | Lateral patella | 1.2 | Left | Lateral retinacular release and medial plication |
| 5 | Lateral patella | 1.2 | Right | Lateral retinacular release, partial medial meniscal meniscectomy, Fulkerson osteotomy |
| 6 | Trochlea | 1.4 | Right | |
| 7 | Medial patella and trochlea | 1.4 | Left | ACL reconstruction with hamstrings |
| 8 | Trochlea | 1.3 | Left | Partial sinovectomy |
| 9 | Trochlea and lateral patella | 1.2 | Right | |
| 10 | Trochlea | 0.9 | Left | |
| MF | ||||
| 1 | Trochlea | 1.5 | Right | ACL reconstruction with hamstrings, lateral meniscal repair |
| 2 | Trochlea | 1.2 | Left | Lateral retinacular release and medial plication |
| 3 | Trochlea | 1.3 | Right | |
| 4 | Medial patella | 1.1 | Right | Medial meniscal repair |
| 5 | Lateral patella | 1.2 | Right | Lateral retinacular release, medial plication and Fulkerson osteotomy |
| 6 | Lateral patella | 1.2 | Left | Lateral retinacular release and medial plication |
| 7 | Trochlea | 1 | Left |
MACI = matrix autologous chondrocyte implantation; MF = microfracture; ACL = anterior cruciate ligament.
Clinical Assessment
Clinically the difference between baseline and 48 months postoperatively for both MACI and MF was significant for Lysholm (P = 0.001, P = 0.001), IKDC-S (International Knee Documentation Committee score) (P = 0.001, P = 0.002), KOOS-P (Knee Injury and Osteoarthritis Outcome score) (P = 0.000, P = 0.002), KOOS-DLA (P = 0.002, P = 0.003), KOOS-Sports/Rec (P = 0.000, P = 0.004), KOOS-QoL (P = 0.000, P = 0.003), KOOS-symptoms (P = 0.001, P = 0.020), and Kujala (P = 0.000, P = 0.01). Tegner values were significant between baseline and 48 months only in MACI (P = 0.008); MF did not show significant difference for this score comparing preoperative to 48 months (P = 0.25). No significant difference was observed between groups for any of the evaluated scores at 4-year follow-up (P < 0.05) ( Table 3 ).
Table 3.
Demographic and Clinical Results at 4-Year Follow-up.
| Group | Patients | Age | lesion (cm2) | Lysholm | Kujala | IKDC-S | KOOS (Symptoms) | KOOS (Pain) | KOOS (Function) | KOOS (Sports) | KOOS (QoL) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MACI | 10 | 36 ± 3.92 | 1.18 ± 0.25 | 89.6 ± 10.61 | 85.4 ± 14.25 | 73.32 ± 16.18 | 19.89 ± 15.88 | 36.89 ± 15.93 | 30.65 ± 25.43 | 42 ± 23.59 | 37.51 ± 25.81 |
| MF | 7 | 39 ± 8.72 | 1.21 ± 0.27 | 75 ± 21.69 | 63.57 ± 17.37 | 61.76 ± 19.18 | 20.08 ± 20.48 | 21.91 ± 13.49 | 20.44 ± 13.37 | 30.71 ± 20.90 | 24.98 ± 10.23 |
MACI = matrix autologous chondrocyte implantation; MF = microfracture; IKDC-S = International Knee Documentation Committee score; KOOS = Knee Injury and Osteoarthritis Outcome score. No significant difference was observed between groups for any of the evaluated scores at 4-year follow-up (P < 0.05).
T2-Mapping: Quantitative Evaluation of Cartilage Repair
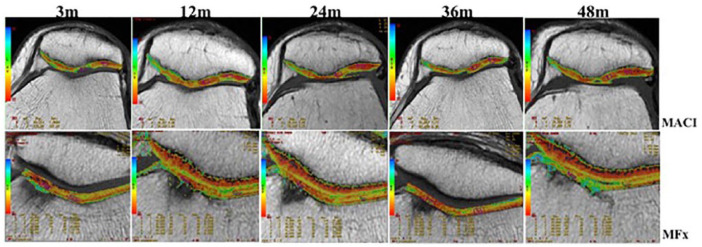
T2-mapping was used to evaluate the quality of the repair tissue after MACI and MF treatments. Patients were followed-up at 3, 12, 24, and 48 months postoperatively and baseline values of healthy cartilage were also compared ( Fig. 6 ; Table 4 ). There was no significant difference at 3, 12, and 24 months between MACI (55, 40.6, and 39.3) and MF (55.3, 47.3, and 50.4) (P > 0.05). However, T2-mapping improved significantly over time in MACI (36.5) compared with MF (48.3) at 48 months (P = 0.005). Moreover, when comparing control values (34.2, 35.7, and 37.2) to repaired tissue at 12-, 24-, and 48-month follow-up, T2 values were not significant different in MACI (40.6, 39.3, and 36.54) (P > 0.05). However, newly formed tissue with MF showed values significant different to native tissue at 3-, 12-, 24-, and 48-month follow-up (55.3, 47.3, 50.4, and 48.3) (P < 0.05) ( Table 5 ).
Figure 6.
Quantitative assessment of repaired cartilage by T2-mapping. Invasive imaging method that provides quantitative values that represents the quality of the repair tissue. Similar behavior was observed at 3 postoperative months with the presence of a high signal intensity in both groups. By 12 and 24 months, treated defects in both techniques had well-defined surface and intermediate signal intensity with more closed values to native cartilage without significant difference between experimental groups. T2-mapping values improved significantly over time in MACI compared to MF at 48 months (P < 0.05). At most MACI sites (above), the chondral defect was completely filled with homogeneous tissue to the expected level of the adjacent articular cartilage.
Table 4.
Quality of Repaired Tissue Evaluated by T2-Mapping through Time.
| Group | 3 Months | 12 Months | 24 Months |
|---|---|---|---|
| Control | */** 36.61 ± 5.08 | *34.28 ± 9.39 | *35.72 ± 5.45 |
| MACI | **55.00 ± 6.55 | 40.60 ± 6.40 | **39.35 ± 5.54 |
| MF | *55.31 ± 17.43 | *47.31 ± 9.61 | */** 50.44 ± 8.31 |
MACI = matrix autologous chondrocyte implantation; MF = microfracture. There was no significant difference at 3, 12, and 24 months between MACE and MF (P > 0.05). At 48 months MACI showed significant better results than MF (P < 0.05). When comparing control values with repaired tissue at 12-, 24-, and 48-month follow-up, no significant difference was observed (P > 0.05). Repair tissue with MF technique showed significantly inferior quality than control cartilage at all times of follow-up (3, 12, 24, and 48 months) (P < 0.05).
/**Significant difference (P < 0.05).
Table 5.
Patients’ Characteristics at the End of Follow-up.
| Group | Patients | Age | lesion Size (cm2) | Lysholm | Kujala | IKDC-S | KOOS (Symptoms) | KOOS (Pain) | KOOS (Function) | KOOS (Sports) | KOOS (QoL) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| MACI | 10 | 36 ± 3.92 | 1.18 ± 0.25 | 89.6 ± 10.61 | 85.4 ± 14.25 | 73.32 ± 16.18 | 19.89 ± 15.88 | 36.89 ± 15.93 | 30.65 ± 25.43 | 42 ± 23.59 | 37.51 ± 25.81 |
| MF | 7 | 39 ± 8.72 | 1.21 ± 0.27 | 75 ± 21.69 | 63.57 ± 17.37 | 61.76 ± 19.18 | 20.08 ± 20.48 | 21.91 ± 13.49 | 20.44 ± 13.37 | 30.71 ± 20.90 | 24.98 ± 10.23 |
MACI = matrix autologous chondrocyte implantation; MF = microfracture; IKDC-S = International Knee Documentation Committee score; KOOS = Knee Injury and Osteoarthritis Outcome score. No significant difference was found in clinical evaluations between groups at 4-year follow-up.
MOCART Score
MOCART score was used only preoperatively and at 48-month follow-up MRI of the patients to do a qualitative assessment of the cartilage repair, with a mean of 67.5 for the MF group and a mean of 73 for MACI; we obtained from a t test a mean difference of 5.5 (−14.9 to 3.9 95% confidence interval; P > 0.5), showing no significant difference between groups.
Second Look
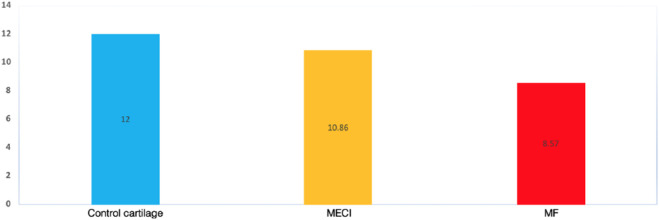
The repair tissue assessed with ICRS classification was significantly better in MACI (10.86 ± 0.38) compared with MF (8.57 ± 2.07) (p = 0.01) (Graph 1).
Graph 1.
ICRS score evaluation at second look.
Discussion
In our study, we observed similar behavior in both groups in the initial T2-mapping MRI (3 postoperative months) with the presence of a high signal intensity (MACI: 55 and MF: 55.3) and features of edema-like imaging probably because of the presence of thin and poor organized immature tissue. By 1 to 2 years after surgery, treated defects in both techniques evaluated with T2-mapping had well-defined surface and intermediate signal intensity with more closed values to native cartilage in MACI (40.6 and 39.3) compared with MF (47.3 and 50.4) but without significant difference in the quality of the repair tissue between groups. However, when comparing control values to repaired tissue at 12 and 24 months (34.2 and 35.7, respectively), T2 values were not significant different in MACI (40.6 and 39.3) (P > 0.05) but there was significant difference between control tissue and MF at those follow-up times. At most MACI sites, the chondral defect was completely filled with homogeneous tissue to the expected level of the adjacent articular cartilage. MOCART score showed no difference between groups, and a greater sample is needed.
Surgical treatment for cartilage lesions in the PFJ is recommended when patient has persistent symptoms despite conservative treatment. Surgical options to repair cartilage in the PFJ vary from MF, ACL, and osteochondral transplantation. MF is considered to have the best results in young patients (<40 years old) with small lesions of the femoral condyles. However, in PFJ MF is considered technically challenge due to trouble obtaining a perpendicular angle of approach arthroscopically. Additionally, the outcomes following MF tend to decline with time probably because of the inferior characteristics of the repair tissue, the poor integration with the surrounding cartilage, and incomplete defect filling. Mithoefer et al. reviewed 28 studies about this technique demonstrating that in all there is a symptomatic improvement during the first 24 postoperative months. However, 7 of the studies reported deterioration of the functional outcomes in 47% to 80% of patients between 18 and 36 months. 16 In our study significant clinical improvement was observed since the first 3 months posttreatment with maintenance of good values at 4-year follow-up showing similar results than MACI in the clinical assessment but not in the quality of repair tissue evaluated by T2-mapping.
Different to MF that is considered a cartilage repair technique, ACI employs tissue-engineering cell-based therapy to regenerate cartilage. This technique has shown good to excellent long-term results in the cartilage repair field. However, outcomes in patellar and trochlear lesions have had mixed results. Brittberg et al. described cases of 23 patients, of whom 7 had patellar-based lesions, and of those, 2 had excellent results (28%), 3 had good results, and 2 had poor results. 19 In another study, the same authors reported a follow-up of 81% of these patients had good-to-excellent results at 2 years, and 83% at 5- to 11-year follow-up. 12 Peterson et al. reported 65% satisfactory results in the treatment of isolated cartilage lesions in the patella with ACI; however, when ACI was combined with unloading tibial tubercle osteotomy (AMZ) the authors observed good to excellent results in 85% of the patients. 20 ACI in combination with AMZ has been shown to have superior outcomes, as compared with ACI alone, with 86% of patients having good-to-excellent results following the combined procedure versus 55% of the patients treated with ACI alone.21-23
In this study, 40% and 42% of patients had additional unloading or realignment procedures for the patella in the MACI and MF techniques, respectively. Also, 14% of patients in every group had an ACL reconstruction without significant difference in clinical results compared with those patients that did not present ACL instability (P > 0.05). Other reported concomitant pathologies were meniscal lesions in 28% in the MF group. The group analysis showed a relevant difference between chondral lesion sites in each of the groups, because the patella being a more easily accessed site for the MACI procedure than for the MF procedure.
The need to address associated pathological conditions such as tibiofemoral axis misalignment, patellofemoral maltracking, and ligamentous insufficiency, in a previous or concomitant cartilage repair procedure, is widely recognized.24,25 Articular cartilage restoration techniques with concomitant correction of tibiofemoral axis misalignment provide greater survival at medium- and long-term follow-up.25,26 Similarly, concomitant patellofemoral maltracking correction reduces overloading of the lateral PFJ and therefore reduces the risk of future cartilage injuries. 8
MRI by T2-mapping provides a useful evaluation of the morphologic status or repaired cartilage throughout the time. Compared with other techniques, such as biopsy and second arthroscopic view, T2-mapping is a noninvasive imaging method that provides quantitative values that represents the quality of the repair tissue. Following cartilage repair treatments, MRI can help not only to evaluate quantitatively the quality of the repaired cartilage but also to define the defect filling, the integration of the newly formed tissue to the adjacent native cartilage, and to detect failures as delamination or hypertrophy.
Cartilage T2 quantification is a promising marker for cartilage matrix biochemistry and has been applied in the largest multicenter studies of osteoarthritis with the effort of the Osteoarthritis Initiative gruop. 27 Changes in the signal are dominated by the hydration and collagen fibers and may not be sensitive to subtle changes of PG loss. T2 relaxation times correlate with the degree of organization of collagen fibers: shorter values are recorded in the deep cartilage zones, where collagen is highly organized, and long ones in the transitional zone, where it is less organized, 28 and also it has been shown to be correlated to the integrity of the collagen matrix, although this relationship may be confounded by other variables. 29 T2-mapping measures the collagen component of the cartilage extracellular matrix by assessing the changing interactions between water and collagen molecules and can detect zonal variations in articular cartilage, like injury or degradation.30,31
In cartilage repair tissue, histologically validated animal studies report this increase in zonal T2 as an indicator of the formation of hyaline or “hyaline-like” cartilage.32,33 This mode of imaging was correlated with histological degeneration, and it may be a good biomarker for osteoarthritis in human articular cartilage. However, the is a weak correlation (ρ = 0.313). 16
MOCART score was specifically designed for the analysis of cartilage repair after ACI 34 as an alternative to whole-knee scoring systems designed to assess the severity and progression of osteoarthritis, such as the Whole-Organ Magnetic Resonance Imaging Score (WORMS). 35
In terms of successful treatment of cartilage repair in the PFJ, we conclude that MF and MACI provide significant good results in clinical assessment through time. The quality of repair tissue evaluated by T2-mapping showed better results at final follow-up time (4 years) in MACI compared with MF. However, T2-mapping values in newly formed cartilage in the MACI group were similar to those control values since 12-month follow-up. Although knee scores in both groups resulted in significant difference compared to preoperative values, it is evident that MACI enhances the formation of hyaline-like tissue with relaxation time values evaluated by T2-mapping very close to those of the native tissue and, more important, maintain more stable values through the time compared to the MF procedure.
Limitations
The limitations in this study are the reduced number of patients and the concomitant lesions presented, adding confounding factors to clinical findings. Clinical outcome, as a secondary objective, cannot be adjudicated only to chondral repair in patients with other lesions.
Conclusions
Clinically both techniques improved significantly over time. However, T2-mapping assessment showed that the MACI technique improves significantly since 12 months postoperatively and maintains stable values compared with native cartilage until 48-month follow-up; however, MOCART score showed a difference in favor of MACI but without statistical significance.
Footnotes
Acknowledgments and Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: Ethical approval for this study was obtained from Ethics in Research Committee and Institutional Research Committee (INR 01/11).
Informed Consent: Written informed consent was obtained from all subjects before the study.
References
- 1. Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31 516 knee arthroscopies. Arthroscopy. 1997;13:456-60. [DOI] [PubMed] [Google Scholar]
- 2. Aroen A, Løken S, Heir S, Avlik E, Ekeland A, Granlund OG, et al. Articular cartilage lesions in 993 consecutive knee arthroscopies. Am J Sports Med. 2004;32:211-5. [DOI] [PubMed] [Google Scholar]
- 3. Hjelle K, Solheim E, Strand T, Muri R, Brittberg M. Articular cartilage defects in 1000 knee arthroscopies. Arthroscopy. 2002;18:730-4. [DOI] [PubMed] [Google Scholar]
- 4. Kaplan LD, Schurhoff MR, Selesnick H, Thorpe M, Uribe JW. Magnetic resonance imaging of the knee in asymptomatic professional basketball players. Arthroscopy. 2005;21:557-61. [DOI] [PubMed] [Google Scholar]
- 5. Strauss EJ, Galos DK. The evaluation and management of cartilage lesions affecting the patellofemoral joint. Curr Rev Musculoeskelet Med. 2013;6(2):141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Filardo G, Kon E, Andriolo L, Di Martino A, Zaffagnini S, Marcacci M. Treatment of “patellofemoral” cartilage lesions with matrix-assisted autologous chondrocyte transplantation: a comparison of patellar and trochlear lesions. Am J Sports Med. 2014;42(3):626-34. [DOI] [PubMed] [Google Scholar]
- 7. Astur DC, Arliani GG, Binz M, Astur N, Kaleka CC, Amaro JT, et al. Autologous osteochondral transplantation for treating patellar chondral injuries: evaluation, treatment, and outcomes of a two-year follow-up study. J Bone Joint Surg. 2014;96(10):816-23. [DOI] [PubMed] [Google Scholar]
- 8. Kramer D, Kocher M. Management of patellar and trochlear chondral injuries. Oper Tech Orthop. 2007;17(4):234-43. [Google Scholar]
- 9. Meister K, Cobb A, Bentley G. Treatment of painful articular cartilage defects of the patella by carbon-fibre implants. J Bone Joint Surg Br. 1998;80(6):965-70. [DOI] [PubMed] [Google Scholar]
- 10. Niemeyer P, Steinwachs M, Erggelet C, Kreuz PC, Kraft N, Köstler W, et al. Autologous chondrocyte implantation for treatment of retropatellar cartilage defects: clinical results referred to defect localisation. Arch Orthop Trauma Surg. 2008;128:1223-31. [DOI] [PubMed] [Google Scholar]
- 11. Pascual-Garrido C, Slabaugh MA, L’Heureux DR, Friel NA, Cole BJ. Recommendations and treatment outcomes for patellofemoral articular cartilage defects with autologous chondrocyte implantation: prospective evaluation at average 4-year follow-up. Am J Sports Med. 2009;37(Suppl 1):33S-41S. [DOI] [PubMed] [Google Scholar]
- 12. Brittberg MM, Peterson LL, Sjögren-Jansson EE, Tallheden T, Lindahl A. Articular cartilage engineering with autologous chondrocyte transplantation. A review of recent developments. J Bone Joint Surg Am. 2003;85-A(Suppl 3):109-15. [DOI] [PubMed] [Google Scholar]
- 13. Knutsen G, Engebretsen L, Ludvigsen TC, Drogset JO, Grøntvedt T, Solheim E, et al. Autologous chondrocyte implantation compared with microfracture in the knee: A randomized trial. J Bone Joint Surg. 2004;86:455-64. [DOI] [PubMed] [Google Scholar]
- 14. Steadman JR, Briggs KK, Rodrigo JJ, Kocher MS, Gill TJ, Rodkey WG. Outcomes of microfracture for traumatic chondral defects of the knee: average 11-year follow-up. Arthroscopy. 2003;19;477-84. [DOI] [PubMed] [Google Scholar]
- 15. Kon E, Gobbi A, Filardo G, Delcogliano M, Zaffagnini S, Marcacci M. Arthroscopic second-generation autologous chondrocyte implantation compared with microfracture for chondral lesions of the knee: prospective nonrandomized study at 5 years. Am J Sports Med. 2009;37:33-41. [DOI] [PubMed] [Google Scholar]
- 16. Mithoefer K, McAdams T, Williams RJ, Kreuz PC, Mandelbaum BR. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: an evidence-based systematic analysis. Am J Sports Med. 2009;37(10):2053-63. [DOI] [PubMed] [Google Scholar]
- 17. Kim T, Min BH, Yoon SH, Kim H, Park S, Lee HY, et al. An in vitro comparative study of T2 and T2* mappings of human articular cartilage at 3-Tesla MRI using histology as the standard of reference. Skeletal Radiol. 2014;43(7):947-54. [DOI] [PubMed] [Google Scholar]
- 18. Masri M, Lombardero G, Velasquillo C, Martínez V, Neri R, Villegas H, et al. Matrix-encapsulation cell-seeding technique to prevent cell detachment during arthroscopic implantation of matrix-induced autologous chondrocytes. Arthroscopy. 2007;23(8):877-83. [DOI] [PubMed] [Google Scholar]
- 19. Brittberg M, Lindahl A, Nilsson A, Ohlsson C, Isaksson O, Peterson L. Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med. 1994;331(14):889-95. [DOI] [PubMed] [Google Scholar]
- 20. Peterson L, Minas T, Brittberg M, Nilsson A, Sjögren-Jansson E, Lindahl A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000;(374):212-34. [DOI] [PubMed] [Google Scholar]
- 21. Vanlauwe JJ, Claes T, Van Assche D, Bellemans J, Luyten FP. Characterized chondrocyte implantation in the patellofemoral joint: an up to 4-year follow-up of a prospective cohort of 38 patients. Am J Sports Med. 2012;40(8):1799-807. [DOI] [PubMed] [Google Scholar]
- 22. Henderson IJ, Lavigne P. Periosteal autologous chondrocyte implantation for patellar chondral defect in patients with normal and abnormal patellar tracking. Knee. 2006;13(4):274-9. [DOI] [PubMed] [Google Scholar]
- 23. Farr J, 2nd. Autologous chondrocyte implantation and anteromedialization in the treatment of patellofemoral chondrosis. Orthop Clin North Am. 2008;39(3):329-35. [DOI] [PubMed] [Google Scholar]
- 24. Takeda H, Nakagawa T, Nakamura K, Engebretsen L. Prevention and management of knee osteoarthritis and knee cartilage injury in sports. Br J Sports Med. 2011;45(4):304-9. [DOI] [PubMed] [Google Scholar]
- 25. Harris JD, McNeilan R, Siston RA, Flanigan DC. Survival and clinical outcome of isolated high tibial osteotomy and combined biological knee reconstruction. Knee. 2013;20(3):154-61. [DOI] [PubMed] [Google Scholar]
- 26. Bauer S, Khan RJ, Ebert JR, Robertson WB, Breidahl W, Ackland TR, et al. Knee joint preservation with combined neutralising high tibial osteotomy (HTO) and matrix-induced autologous chondrocyte implantation (MACI) in younger patients with medial knee osteoarthritis: a case series with prospective clinical and MRI follow-up over 5 years. Knee. 2012;19(4):431-9. [DOI] [PubMed] [Google Scholar]
- 27. Li X, Majumdar S. Quantitative MRI of articular cartilage and its clinical applications. J Magn Reson Imaging. 2013;38(5):991-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ronga M, Angeretti G, Ferraro S, De Falco G, Genovese EA, Cherubino P. Imaging of articular cartilage: current concepts. Joints. 2014;2(3):137-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Blumenkrantz G, Majumdar S. Quantitative magnetic resonance imaging of articular cartilage in osteoarthritis. Eur Cell Mater. 2007;13:76-86. [DOI] [PubMed] [Google Scholar]
- 30. Jazrawi LM, Alaia MJ, Chang G, Fitzgerald EF, Recht MP. Advances in magnetic resonance imaging of articular cartilage. J Am Acad Orthop Surg. 2011;19(7):420-9. [DOI] [PubMed] [Google Scholar]
- 31. Soellner ST, Goldmann A, Muelheims D, Welsch GH, Pachowsky ML. Intraoperative validation of quantitative T2 mapping in patients with articular cartilage lesions of the knee. Osteoarthritis Cartilage. 2017;25(11):1841-9. [DOI] [PubMed] [Google Scholar]
- 32. White LM, Sussman MS, Hurtig M, Probyn L, Tomlinson G, Kandel R. Cartilage T2 assessment: differentiation of normal hyaline cartilage and reparative tissue after arthroscopic cartilage repair in equine subjects. Radiology. 2006;241:407-14. [DOI] [PubMed] [Google Scholar]
- 33. Watrin-Pinzano A, Ruaud JP, Cheli Y, Gonord P, Grossin L, Bettembourg-Brault I, et al. Evaluation of cartilage repair tissue after biomaterial implantation in rat patella by using T2 mapping. MAGMA. 2004;17:219-28. [DOI] [PubMed] [Google Scholar]
- 34. Marlovits S, Singer P, Zeller P, Mandl I, Haller J, Trattnig S. Magnetic resonance observation of cartilage repair tissue (MOCART) for the evaluation of autologous chondrocyte transplantation: determination of interobserver variability and correlation to clinical outcome after 2 years. Eur J Radiol. 2006;57(1):16-23. [DOI] [PubMed] [Google Scholar]
- 35. Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12(3):177-90. [DOI] [PubMed] [Google Scholar]