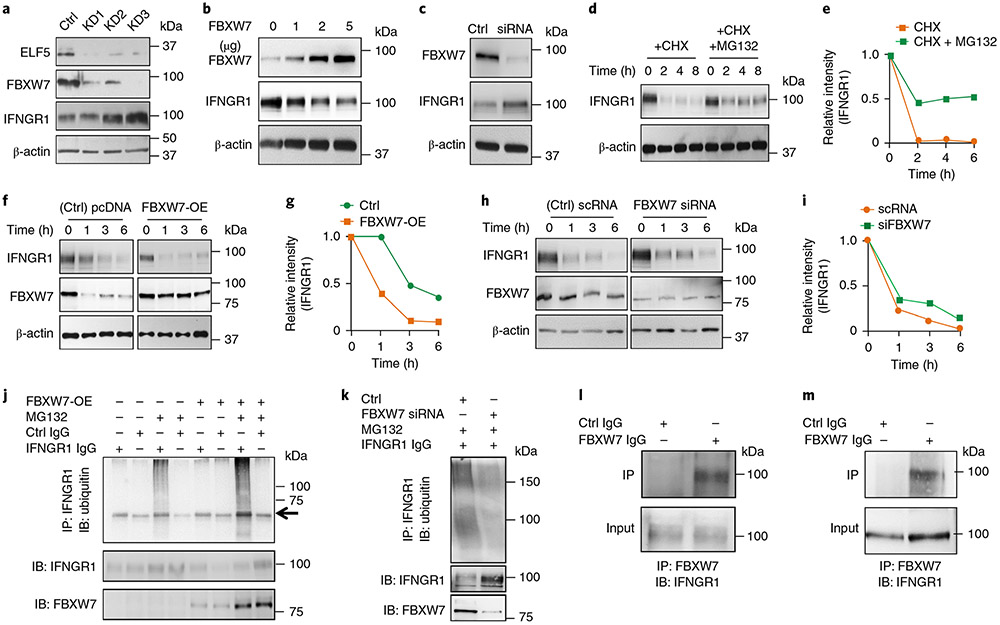
Fig. 3 ∣. ELF5 regulates FBXW7-mediated destabilization of IFNGR1 in TNBC.
a, Stable knockdown of ELF5 in MX1 cells decreases FBXW7 levels and increases IFNGR1 protein expression. Three independent shRNA sequences were used to knockdown ELF5 (KD1, KD2 and KD3). b, Overexpression of FBXW7 in LM2 cells decreases IFNGR1 protein expression in a dose-dependent manner. c, Silencing FBXW7 in MX1 cells by siRNA58 causes an increase in IFNGR1 levels. d,e, Western blot (d) and quantification of the results (e), showing degradation of IFNGR1 in LM2 cells upon treatment with CHX and rescue upon co-treatment with MG132, which prevents proteasomal degradation. f,g, Overexpression of FBXW7 in LM2 cells enhances degradation of IFNGR1, as shown by western blot (f) and quantification of the results (g). h,i, Knockdown of FBXW7 in MX1 cells delays CHX-mediated degradation of IFNGR1 protein, as shown by western blot (h) and quantification of the results (i). j, LM2 cells were transfected with FBXW7 overexpressing plasmid followed by treatment with MG132 after 48 h. Immunoprecipitation was performed with anti-IFNGR1 antibody followed by western blot with ubiquitin antibody. The results show that polyubiquitination of IFNGR1 was increased upon overexpression of FBXW7. The arrow shows a heavy chain. k, MX1 cells were transfected with FBXW7 siRNA followed by treatment with MG132 after 48 h. Immunoprecipitation was performed with anti-IFNGR1 antibody followed by western blot with ubiquitin antibody. The results show that polyubiquitination of IFNGR1 was decreased upon knockdown of FBXW7. l,m, LM2 (l) and MX1 (m) cells were subjected to endogenous immunoprecipitation using FBXW7 antibody followed by probing with IFNGR1 antibody. Direct interaction between FBXW7 and IFNGR1 was observed in both sets of TNBC cells. All experiments in a-m were repeated three times. Uncropped western blot images are provided as source data. Ctrl, control; IB, immunoblotting; IP, immunoprecipitation; pcDNA, plasmid cloning DNA; scRNA, small conditional RNA.