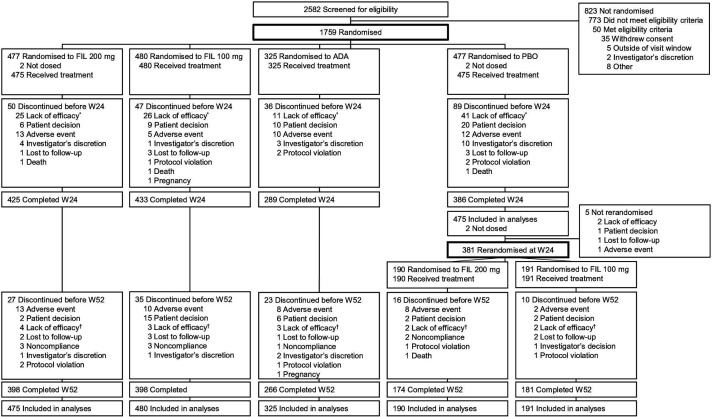
Figure 1.
Patient disposition. *23 (4.8%) patients treated with filgotinib 200 mg, 29 (6.0%) patients treated with filgotinib 100 mg, 13 (4.0%) patients treated with adalimumab, and 41 (8.6%) patients treated with placebo did not have adequate response to treatment per protocol at week 14. †3 (0.7%) patients treated with filgotinib 200 mg, 2 (0.5%) patients treated with filgotinib 100 mg, 3 (1.0%) patients treated with adalimumab, 0 patient treated with placebo and rerandomised to filgotinib 200 mg at week 24, and 4 (2.2%) patients treated with placebo and rerandomised to filgotinib 100 mg at week 24 failed to maintain response to treatment per protocol after week 30. ADA, adalimumab; FIL, filgotinib; PBO, placebo; W, week.